Abstract
Background: Premature infants are deprived of prenatal accumulation of brain docosahexaenoic acid [DHA (22:6n–3)], an omega-3 fatty acid [ω-3 FA (n–3 FA)] important for proper development of cognitive function. The resulting brain DHA deficit can be reversed by ω-3 FA supplementation.
Objective: The objective was to test whether there is a critical period for providing ω-3 FA to correct cognitive deficits caused by developmental ω-3 FA deprivation in mice.
Methods: Twelve timed-pregnant mice [embryonic day 14 (E14), C57/BL6NCr] were fed an ω-3 FA–deficient diet containing 0.04% α-linolenic acid [ALA (18:3n–3)], and their offspring were fed the same deficient diet (Def group) or changed to an ω-3 FA–adequate diet containing 3.1% ALA at 3 wk, 2 mo, or 4 mo of age. In parallel, 3 E14 pregnant mice were fed the adequate diet and their offspring were fed the same diet (Adeq group) throughout the experiment. Brain FA composition, learning and memory, and hippocampal synaptic protein expression were evaluated at 6 mo by gas chromatography, the Morris water maze test, and western blot analysis, respectively.
Results: Maternal dietary ω-3 FA deprivation decreased DHA by >50% in the brain of their offspring at 3 wk of age. The Def group showed significantly worse learning and memory at 6 mo than those groups fed the adequate diet. These pups also had decreased hippocampal expression of postsynaptic density protein 95 (43% of Adeq group), Homer protein homolog 1 (21% of Adeq group), and synaptosome-associated protein of 25 kDa (64% of Adeq group). Changing mice to the adequate diet at 3 wk, 2 mo, or 4 mo of age restored brain DHA to the age-matched adequate concentration. However, deficits in hippocampal synaptic protein expression and spatial learning and memory were normalized only when the diet was changed at 3 wk.
Conclusion: Developmental deprivation of brain DHA by dietary ω-3 FA depletion in mice may have a lasting impact on cognitive function if not corrected at an early age.
Keywords: omega-3 fatty acid, n-3 PUFA, cognitive deficits, synaptic protein, brain, development
Introduction
The brain contains a higher percentage of lipids than any other organ in the body. Lipids account for ∼60% of the brain’s dry weight, ∼40% of which are long-chain PUFAs (1). DHA (22:6n–3) is the major omega-3 (ω-3) PUFA in the brain and retina and is important for signal transduction, neurotransmission, and neurogenesis (2). DHA and its metabolite N-docosahexaenoylethanolamine promote neurogenesis, neurite growth, synaptogenesis, synaptic protein expression, and synaptic function (3–6). Studies with rodents indicate that dietary depletion of ω-3 FA results in DHA deficits in the brain and impairs learning and memory (7, 8).
The brain growth spurt occurs from the third trimester of pregnancy throughout the first 2 y of life in humans and during the first 3–4 wk of neonatal life in rats and mice (9). Fetal brain DHA accretion is highest during the last trimester of pregnancy in humans (10), with a rate of ∼14.5 mg/wk (11). In rodents, brain DHA content increases by 10-fold (from 0.64 to 6.6 mg/brain, at ∼2 mg/wk) during the first 3 wk of neonatal life (12). The sharp increases in brain DHA accretion coincide with the growth spurt when the extensive axonal and dendritic outgrowth and development of synaptic connections are made (10, 13, 14), suggesting the importance of having an optimal amount of DHA in the maternal circulation during the perinatal period in both humans and rodents.
Premature newborns are deprived of prenatal DHA accretion, and there is a direct correlation between DHA concentrations and infant gestational age; the shorter the duration of the pregnancy, the lower the DHA concentration (15, 16). Thus, preterm infants—especially very-low-birth-weight infants—are at high risk of DHA deficiency. The DHA deficit persists or worsens postnatally as a result of decreased adipose tissue stores, limited ability to synthesize DHA from precursor FAs, and poor nutritional provision of preformed DHA (15, 16), making these preterm infants vulnerable to suboptimal development of DHA-dependent brain function. Indeed, a previous report showed that very-low-birth-weight infants are at an increased risk of developmental disorders and learning disabilities, whereas DHA supplementation through breast milk or formula significantly improved developmental scores at 33 mo of corrected age and resulted in a slight decrease in the proportion of children with a severe mental delay at 18 mo (16).
Similarly, dietary deficiency of ω-3 FA leads to reduced brain DHA in rodents, but its concentrations can be normalized within 2 mo of DHA supplementation (17). Moreover, it has been reported that cognitive deficits in DHA-deficient rats are reversible and can be ameliorated by supplementation of ω-3 FA with no apparent critical period of supplementation (18). Nevertheless, other studies indicate that the age at which ω-3 FA supplementation begins affects the functional outcomes associated with ω-3 FA deficiency, such as retinal responses (19) and arterial blood pressure (20).
Synaptic protein expression is crucial for synaptogenesis during development (21). ω-3 FA deficiency decreases the proteins that mediate synaptic neurotransmission and maintain synapse integrity in rats (22) and mice (4, 23, 24). The resulting synaptic proteome changes are also associated with changes in cognitive function (23), suggesting that synaptic protein expression is a likely mechanism for ω-3 FA–dependent cognitive changes in rodent models. Furthermore, age-dependent alteration in the expression of these synaptic proteins has also been observed (23, 25, 26), posing a possibility that there is a critical period for ω-3 FA supplementation to improve cognition. Nevertheless, to our knowledge, few studies have investigated the effects of ω-3 FA depletion and replenishment in this context.
This study was designed to investigate whether there is a critical postnatal period to initiate ω-3 FA supplementation for the recovery of deficits in synaptic protein expression and cognitive function caused by ω-3 FA deficiency. This study examined brain FA composition and the expression of hippocampal proteins involved in synaptic plasticity in relation to learning and memory performance.
Methods
Experimental diets and animals.
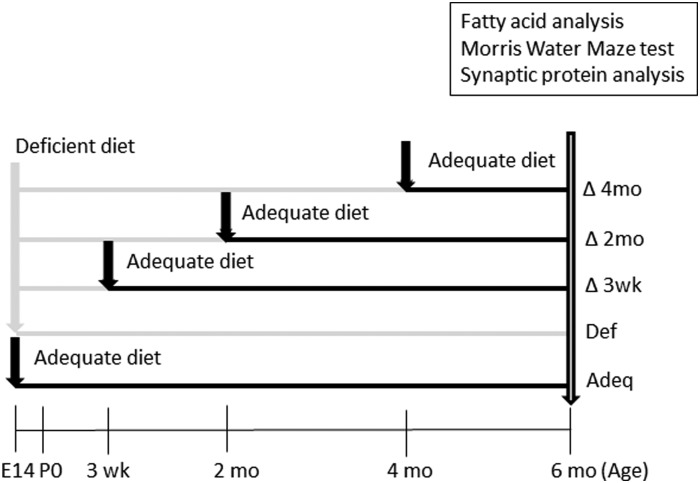
The 2 experimental diets used in this study were ordered from Dyets Inc. The ω-3 FA–adequate diet contained 100 g fat/kg diet from tocopherol-stripped safflower oil, flaxseed oil, and hydrogenated coconut oil. The lipids in the ω-3 FA–deficient diet were tocopherol-stripped safflower oil and hydrogenated coconut oil. Total caloric and nonlipid components of the diets were similar (Supplemental Table 1). The ω-3 FA–adequate diet contained 3.1 wt% of the essential ω-3 FA α-linolenic acid [ALA (18:3n–3)] (3.1/100 g fat) compared with 0.04 wt% in the ω-3 FA–deficient diet (Supplemental Table 2). Figure 1 shows the experimental design of this study. Fifteen timed-pregnant C57BL/6NCr mice ordered from Charles River were housed in individual cages with a 12-h light:dark cycle. Food and water were provided ad libitum. The pregnant dams were randomly designated to be in 1 of the 5 dietary groups a priori. At embryonic day 14, 3 pregnant mice were fed the ω-3 FA–adequate diet and 12 pregnant mice were fed the ω-3 FA–deficient diet. Litters from the dams fed the ω-3 FA–adequate diet were weaned and maintained with the same diet (Adeq group) until 6 mo of age. Of offspring from the 12 dams fed the ω-3 FA–deficient diet, litters from 3 dams (selected randomly) were weaned to the ω-3 FA–adequate diet (∆3 wk group). Those from the remaining 9 dams were weaned to the ω-3 FA–deficient diet. Of these, the litters from 3 dams were changed to the ω-3 FA–adequate diet at 2 mo of age (∆2 mo group) and the litters from 3 dams were changed to the ω-3 FA–adequate diet at 4 mo of age (∆4 mo group). The diet of the litters from the remaining 3 dams was not changed [i.e., they were fed the ω-3 FA–deficient diet throughout the experiment (Def group)]. At 6 mo of age, learning and memory was assessed for pups in all experimental groups by the Morris water maze (MWM) test. After the test, the mice were anesthetized using isoflurane and perfused with PBS, and their brains were removed and randomly allocated for the analysis of FA composition and hippocampal synaptic protein expression. All experiments were performed in accordance with the guiding principles for the care and use of animals approved by the National Institute on Alcohol Abuse and Alcoholism (LMS-HK-13).
FIGURE 1.
Experimental design. Twelve timed-pregnant mice on E14 were fed an ω-3 FA–deficient diet and their pups were either fed the ω-3 FA–deficient diet during the entirety of the study (Def) or changed to an ω-3 FA–adequate diet at postnatal age 3 wk (Δ3 wk), 2 mo (Δ2 mo), or 4 mo (Δ4 mo). In parallel, 3 E14 pregnant mice were fed an ω-3 FA–adequate diet throughout pregnancy and lactation and their pups were given ω-3 FA–adequate diet throughout the experiment (Adeq). Brains were randomly collected from 3 pups of dams fed either diet for FA analysis from representative mice at 3 wk and 6 mo of age. Behavioral tests to assess hippocampal-dependent spatial learning and memory were conducted at 6 mo of age, and brains were collected for hippocampal synaptic protein expression analysis and FA analysis (n = 3 each) after the Morris water maze test. Adeq, mice fed an ω-3 FA–adequate diet; Def, mice fed an ω-3 FA–deficient diet; E14, embryonic day 14; P0, day of birth.
FA analysis.
Mouse brains were collected at 3 wk and at 6 mo after transcardiac perfusion with chilled PBS under isoflurane anesthesia. Lipids from tissue homogenates were extracted according to the Bligh and Dyer method (27) in the presence of heneicosanoic acid (21:0) as an internal standard; after transmethylation, FA analysis was performed by GC as described previously (28). Briefly, aliquots of lipid extracts were transmethylated by incubation with BF3/methanol (14% wt:vol) at 100°C for 2 h under a nitrogen atmosphere. FA methyl esters were extracted with hexane and then analyzed using a Hewlett Packard 5890 gas chromatograph equipped with a flame ionization detector (Palo Alto) and a fused-silica DB-FFAP capillary column (30 m · 0.25 mm internal diameter, 0.25 lm; J&W Scientific). The oven temperature was programmed from 130°C to 180°C at 4°C/min, from 180°C to 215°C at 1°C/min, and then raised to 245°C at 30°C/min, with a final hold for 15 min. The injector and detector temperatures were set at 250°C. Hydrogen was used as carrier gas with a linear velocity of 54 cm/s. Individual FAs were identified by comparing the retention times with known FA standards (GLC-411; Nu-Chek Prep). The content of each individual FA was expressed as a weight percentage of the total FA.
MWM test.
The MWM test (29) was performed to evaluate hippocampal-dependent spatial learning and memory as described earlier (30). The test was conducted in a room that had visible cues including posters and objects suspended from the ceiling that were fully or partially visible from the water surface. The investigator (L Lozada, Department of Pediatrics, Walter Reed National Military Medical Center, Bethesda, MD; 2015) stood immobile in one corner of the room and conceivably also served as a cue. The learning trials were conducted for 3 consecutive days with 4 trials/d. The probe trial was conducted 24 h after the last learning trial (on day 4). A 120-cm diameter circular pool was filled with water (∼25°C). The pool was divided into 4 equal-sized virtual quadrants and a 10-cm2 platform was placed in the center of a quadrant submerged ∼0.5 cm under water. Nontoxic white paint was mixed with the water to make it opaque so that the submerged platform was not visible. During each trial, the mouse was gently released in the water facing the wall from the north, southeast, northeast, or south direction and the order was changed randomly every day. The mouse was given a maximum of 60 s in each trial to find the platform. In cases in which the mouse was not able to locate the platform, it was gently guided to the platform and allowed to remain on it for 15 s. The latency to find the platform was used as a measure of spatial learning. After 3 d of learning trials, the platform was removed on the fourth day. Animals were allowed to swim for 30 s and the time spent searching the quadrant where the platform had been placed in the learning trials was used as a measure for spatial memory. The time that each mouse searched within the platform annulus (defined as a circle of ∼30 cm in diameter with the platform position as the center) was also analyzed. ANY-maze video tracking system software (version 4.99 m) was used to collect and analyze the data. The test and data analysis was conducted in a blinded manner.
Western blot analysis.
Brains from mice in the Def, Δ3 wk, Δ2 mo, and Adeq groups (n = 3/group) were homogenized in a lysis buffer containing protease inhibitors (Cell Signaling Technology). Twenty-five micrograms of protein was separated in precast gels by SDS-PAGE. The proteins were transferred to polyvinylidene difluoride membranes (Amersham Pharmacia Biotech), which were then blocked for 15 min in Tris-buffered saline containing 5% skim milk and 0.1% Tween 20 (Bio-Rad). The membranes were incubated in antibody solutions [anti–postsynaptic density protein 95 (PSD-95; rabbit monoclonal), anti–synaptosome-associated protein of 25 kDa (SNAP-25; rabbit monoclonal), and anti–Homer protein homolog 1 (Homer-1) (rabbit polyclonal; Abcam Cambridge)] overnight at 4°C, washed and incubated with HRP-conjugated secondary antibodies for 2 h, washed again, and then incubated in chemiluminescence detection reagent. Luminescence was imaged using the Kodak Gel Logic 440 Imaging System.
Statistical analyses.
GraphPad Prism (version 7; GraphPad Software Inc.) software was used for statistical analyses. Each animal was treated as an independent sample. The results of the FA analysis and relative expression of hippocampal synaptic proteins are presented as means ± SDs. The results of the behavioral test are expressed as means ± SEMs. Data for the learning trials for the water maze were analyzed by repeated-measures 2-factor ANOVA followed by Tukey’s multiple-comparisons test. One-factor ANOVA was used to analyze all of the data for the probe test with the protected Fisher’s least significant difference test for multiple comparisons. This statistical test was used to prevent the variation observed in the probe test from masking small but biologically significant effects. Results of western blot analysis were analyzed by 1-factor ANOVA followed by Dunnett’s multiple-comparisons test with the ω-3 FA–deficient group as the control. The FA concentration in the ω-3 FA–adequate and ω-3 FA–deficient diets and in the brain of 3-wk-old mice fed the adequate or deficient diets was compared using Student’s unpaired t test. FA data at 6 mo were analyzed by 1-factor ANOVA followed by Tukey’s multiple-comparisons test. P < 0.05 was considered statistically significant. The P values presented in the Results refer to the post hoc multiple-comparisons test used for the analyses after 1- or 2-factor ANOVA unless indicated otherwise.
Results
Brain FA composition.
At 3 wk of age, brain DHA content was already significantly lower in the Def than in the Adeq group (P < 0.001) (Table 1). In contrast, the Def group had significantly higher ω-6 docosapentaenoic acid [DPAn-6 (22:5n–6)] content than did the Adeq group (22:5n–6; P < 0.05). The brain concentrations of adrenic acid (22:4n–6; P < 0.01) and arachidonic acid (20:4n–6; P < 0.05) also were slightly but significantly higher in the Def group than in the Adeq group.
TABLE 1.
Brain FA composition of 3-wk-old mice born to dams fed an ω-3 FA–adequate or ω-3 FA–deficient diet from embryonic day 141
| FAs | Adeq | Def |
| Myristic acid (14:0) | 0.7 ± 0.05 | 0.9 ± 0.29 |
| Palmitic acid (16:0) | 22.4 ± 0.73 | 23.2 ± 0.49 |
| Stearic acid (18:0) | 21.3 ± 0.14 | 20.9 ± 1.01 |
| Arachidic acid (20:0) | 0.4 ± 0.02 | 0.4 ± 0.15 |
| Behenic acid (22:0) | 0.4 ± 0.06 | 0.4 ± 0.11 |
| Lignoceric acid (24:0) | 0.5 ± 0.03 | 0.6 ± 0.07 |
| Myristoleic acid (14:1n–5) | 0.3 ± 0.04 | 0.4 ± 0.05 |
| Palmitoleic acid (16:1n–7) | 0.7 ± 0.03 | 0.7 ± 0.04 |
| Oleic acid (18:1n–9) | 14.7 ± 0.88 | 14 ± 0.45 |
| Vaccenic acid (18:1n–7) | 3.7 ± 0.05 | 4.0 ± 0.21 |
| Gondoic acid (20:1n–9) | 1.3 ± 0.28 | 1.1 ± 0.12 |
| Nervonic acid (24:1n–9) | 1.4 ± 0.16 | 1.1 ± 0.19 |
| Linoleic acid (18:2n–6) | 0.4 ± 0.06 | 0.5 ± 0.17 |
| Homo-γ-linolenic acid (20:3n–6) | 0.4 ± 0.12 | 0.3 ± 0.13 |
| Arachidonic acid (20:4n–6) | 10.4 ± 0.58 | 11.7 ± 0.23* |
| Adrenic acid (22:4n–6) | 2.9 ± 0.08 | 3.6 ± 0.22** |
| Docosapentaenoic acid (22:5n–6) | 2.5 ± 1.96 | 8.6 ± 1.32* |
| DHA (22:6n–3) | 15.0 ± 1.56 | 7.1 ± 0.19*** |
Values are wt% presented as means ± SDs (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 relative to the Adeq group. Adeq, mice fed an ω-3 FA–adequate diet; Def, mice fed an ω-3 FA–deficient diet.
The brain concentrations of low DHA, high DPAn-6, and slightly but significantly elevated adrenic acid and arachidonic acid remained in the Def group at 6 mo of age (Table 2). However, the brain concentrations of DHA and DPAn-6 were normalized to those of the Adeq group at 6 mo of age in all groups in which the diet was changed from ω-3 FA deficient to ω-3 adequate (i.e., Δ3 wk, Δ2 mo, and even Δ4 mo; P < 0.001 compared with Def).
TABLE 2.
Brain FA composition in 6-mo-old mice fed an ω-3 FA–deficient diet and switched to an ω-3 FA–adequate diet at different time points compared with mice fed an ω-3 FA–adequate diet from birth1
| Switched to Adeq |
|||||
| FAs | Deficient | ∆3 wk | ∆2 mo | ∆4 mo | Adeq |
| Myristic acid (14:0) | 0.54 ± 0.01 | 0.42 ± 0.04 | 0.44 ± 0.05 | 0.48 ± 0.04 | 0.58 ± 0.06 |
| Palmitic acid (16:0) | 20.6 ± 0.4 | 21.3 ± 0.5 | 21.5 ± 0.2 | 21.7 ± 0.2 | 21.3 ± 0.8 |
| Stearic acid (18:0) | 22.2 ± 0.6 | 21.7 ± 0.2 | 21.6 ± 0.2 | 21.5 ± 0.2 | 21.7 ± 0.4 |
| Arachidic acid (20:0) | 0.30 ± 0.02 | 0.31 ± 0.02 | 0.29 ± 0.03 | 0.27 ± 0.02 | 0.31 ± 0.07 |
| Behenic acid (22:0) | 0.34 ± 0.04 | 0.31 ± 0.03 | 0.34 ± 0.03 | 0.35 ± 0.02 | 0.37 ± 0.06 |
| Lignoceric acid (24:0) | 0.46 ± 0.13a,b | 0.44 ± 0.04a | 0.46 ± 0.08a,b | 0.50 ± 0.02a,b | 0.66 ± 0.05b |
| Palmitoleic acid (16:1n–7) | 0.48 ± 0.09a | 0.61 ± 0.02a,b | 0.58 ± 0.06a,b | 0.66 ± 0.06b | 0.57 ± 0.06a,b |
| Oleic acid (18:1n–9) | 17.0 ± 0.2 | 18.1 ± 0.1 | 17.3 ± 0.7 | 17.0 ± 0.2 | 16.8 ± 1.0 |
| Vaccenic acid (18:1n–7) | 4.25 ± 0.12c | 4.03 ± 0.03c,d | 4.03 ± 0.10c,d | 3.93 ± 0.02c,d | 3.73 ± 0.22d |
| Gondoic acid (20:1n–9) | 1.51 ± 0.14 | 1.71 ± 0.02 | 1.49 ± 0.23 | 1.43 ± 0.05 | 1.58 ± 0.37 |
| Erucic acid (22:1n–9) | 0.17 ± 0.07 | 0.11 ± 0.01 | 0.11 ± 0.03 | 0.13 ± 0.00 | 0.11 ± 0.04 |
| Nervonic acid (24:1n–9) | 1.67 ± 0.21 | 1.48 ± 0.07 | 1.32 ± 0.47 | 1.45 ± 0.21 | 1.63 ± 0.22 |
| Linoleic acid (18:2n–6) | 0.24 ± 0.04 | 0.31 ± 0.05 | 0.32 ± 0.06 | 0.32 ± 0.02 | 0.31 ± 0.03 |
| γ-Linolenic acid (18:3n–6) | 0.08 ± 0.02 | 0.09 ± 0.02 | 0.09 ± 0.02 | 0.09 ± 0.02 | 0.09 ± 0.01 |
| Homo-γ-linolenic acid (20:3n–6) | 0.17 ± 0.01c | 0.32 ± 0.04d | 0.31 ± 0.06d | 0.31 ± 0.02d | 0.31 ± 0.01d |
| Arachidonic acid (20:4n–6) | 11.6 ± 0.1c | 10.4 ± 0.1d | 10.4 ± 0.4d | 10.4 ± 0.1d | 10.4 ± 0.5d |
| Adrenic acid (22:4n–6) | 3.78 ± 0.10e | 2.79 ± 0.02f | 2.82 ± 0.08f | 2.97 ± 0.05f | 2.71 ± 0.13f |
| ω-6 Docosapentaenoic acid (22:5n–6) | 6.17 ± 0.34e | 0.23 ± 0.05f | 0.28 ± 0.02f | 0.54 ± 0.17f | 0.25 ± 0.01f |
| ω-3 Docosapentaenoic acid (22:5n–3) | 0.06 ± 0.11 | 0.11 ± 0.05 | 0.16 ± 0.02 | 0.14 ± 0.08 | 0.16 ± 0.11 |
| DHA (22:6n–3) | 8.20 ± 0.54e | 15.2 ± 0.1f | 16.0 ± 0.7f | 15.6 ± 0.2f | 16.2 ± 1.1f |
Values are wt% presented as means ± SDs (n = 3). Different superscript letters in a row indicate statistical significance reached at the indicated P value compared by 1-factor ANOVA followed by Tukey’s multiple-comparisons test. a,bP < 0.05, c,dP < 0.01, e,fP < 0.001. Adeq, mice fed an ω-3 FA–adequate diet; Def, mice fed an ω-3 FA–deficient diet; ∆2 mo, diet changed at 2 mo of age; Δ3 wk, diet changed at 3 wk of age; ∆4 mo, diet changed at 4 mo of age.
Learning and memory function.
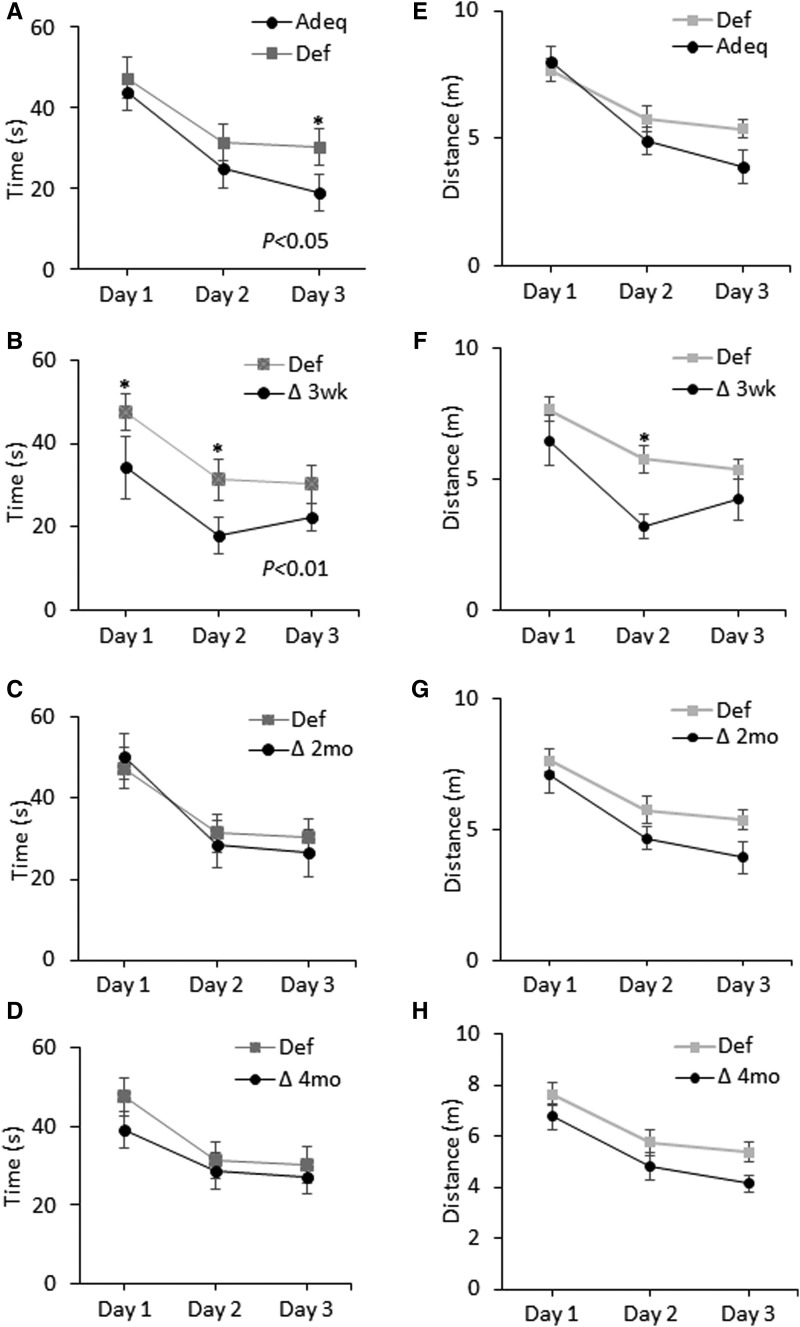
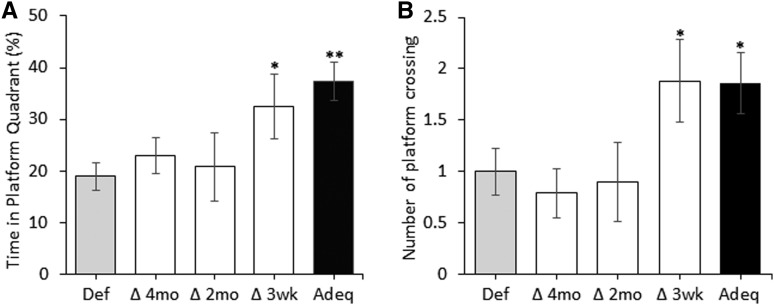
The MWM test was performed to assess hippocampal-dependent spatial learning and memory in mice at 6 mo of age. Figure 2 shows the time and distance traveled to find the platform during the 3-d learning trials. The Def group took significantly longer to reach the platform than the Adeq group (P < 0.05, 2-factor ANOVA: main effect of treatment) (Figure 2A). For the Adeq group, the distance to the platform followed a trend similar to the time to the platform and remained lower for days 2 and 3 but did not reach statistical significance (Figure 2E). The Def group also spent less time in the platform quadrant in the probe trial without the platform than the Adeq group (18.9% ± 2.8% and 37.3% ± 3.74%; P < 0.01) (Figure 3A). Moreover, the Def group made fewer passes through the platform location than the Adeq group (P < 0.05; Figure 3B). ω-3 FA–deficient mice also searched the platform annulus for less time and distance (2.7 ± 0.4 s and 0.57 ± 0.09 m) than did ω-3 FA–adequate mice (5.68 ± 0.97 s and 1.21 ± 0.18 m; P < 0.05 for search time, P < 0.01 for distance) Changing mice to the ω-3 FA–adequate diet at 3 wk resulted in improved learning and memory with significantly shorter latency to find the platform (Figure 2B, P < 0.01, 2-factor ANOVA: main effect of treatment) and a shorter distance to the platform (day 2, P < 0.05) (Figure 2F). This difference was also observed in the probe trial, with the Δ3 wk group searching the platform quadrant longer (P < 0.05) and making more passes through the platform location (P = 0.06) than the Def group (Figure 3B). The Δ3 wk group also searched the platform annulus for a longer time and distance (5.3 ± 1.46 s and 1.12 ± 0.29 m; P = 0.06) than the ω-3 FA–deficient mice (2.7 ± 0.4 s and 0.57 ± 0.09 m; P < 0.05, respectively). Changing mice to the ω-3 FA–adequate diet at 2 or 4 mo did not result in recovery of the learning and memory deficits owing to ω-3 FA deficiency. The time to reach the platform during the learning trials (Figure 2C, D), the time in the platform quadrant (Figure 3A), and the number of platform crossings (Figure 3B) remained similar to those observed for the Def group. These findings indicate that learning and memory deficit persists in ω-3 FA–deficient mice unless they are fed an ω-3 FA–adequate diet early enough (i.e., immediately after weaning).
FIGURE 2.
Effect of the ω-3 FA–adequate diet on learning deficit in ω-3 FA–deficient mice measured at 6 mo of age. (A–D) Latencies to reach the platform across 3 d of learning trials. (E–F) Corresponding distances to the platform. (A and E) Initial groups were Def (n = 18; 9 female and 9 male) or Adeq (n = 14; 7 female and 7 male). (B–H) Mice were changed from the Def group to ∆3 wk (n = 8; 5 female and 3 male) (B and F), Δ2 mo (n = 10; 3 female and 7 male) (C and G), or Δ4 mo (n = 19; 8 female and 11 male) (D and H) groups. Data are presented as means ± SEMs. *P < 0.05 (different between 2 diet groups at the indicated time points). Adeq, mice fed an ω-3 FA–adequate diet; Def, mice fed an ω-3 FA–deficient diet; ∆2 mo, diet changed at 2 mo of age; Δ3 wk, diet changed at 3 wk of age; ∆4 mo, diet changed at 4 mo of age.
FIGURE 3.
Effect of the ω-3 FA–adequate diet on memory deficit in ω-3 FA–deficient mice measured at 6 mo of age. Probe test results of the Morris water maze test measured at 6 mo of age in each diet group. (A) The percentage of time spent in the platform quadrant. (B) The number of platform crossings as an indicator of spatial memory. Groups were as follows: Def, n = 18 (9 female and 9 male); Δ4 mo, n = 19 (8 female and 11 male); Δ2 mo, n = 10 (3 female and 7 male); ∆3 wk, n = 8 (5 female and 3 male); and Adeq, n = 14 (7 female and 7 male). Data are presented as means ± SEMs. *P < 0.05, **P < 0.01 (compared with the ω-3 FA–deficient group). Adeq, mice fed an ω-3 FA–adequate diet; Def, mice fed an ω-3 FA–deficient diet; ∆2 mo, diet changed at 2 mo of age; Δ3 wk, diet changed at 3 wk of age; ∆4 mo, diet changed at 4 mo of age.
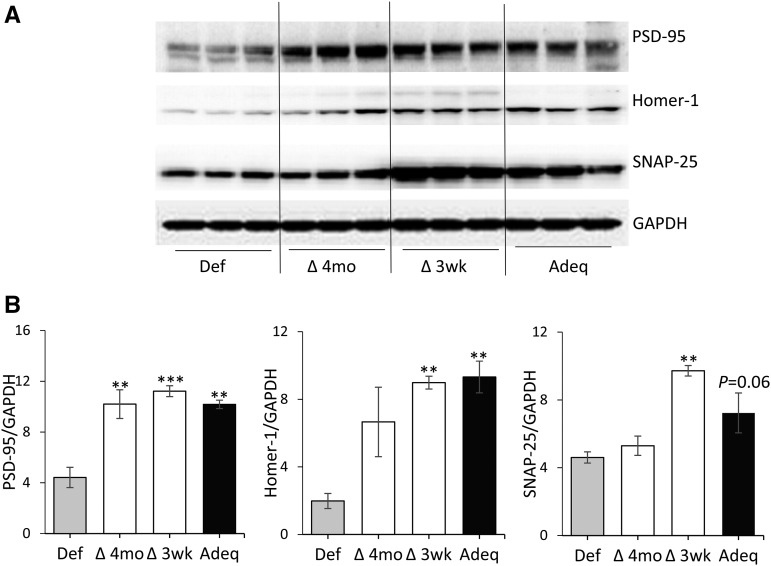
Hippocampal synaptic protein expression.
Figure 4 shows the effects of ω-3 FA status on the expression of synaptic proteins SNAP-25, Homer-1, and PSD-95 in the hippocampus at 6 mo of age. Western blot data obtained from each group of mice are shown in Figure 4A. Quantitative results normalized to GAPDH are shown in Figure 4B. The relative expression of PSD-95 and Homer-1 was significantly increased in the Adeq group, whereas that of SNAP-25 had an increasing trend (P = 0.06) compared with the Def group, as reported earlier (24). The expression of Homer-1 and SNAP-25 reverted to the level found in the Adeq group when ω-3 FA–deficient mice were fed the ω-3 FA–adequate diet at 3 wk of age, but not when the change was delayed until 4 mo of age. As opposed to these results, PSD-95 relative expression increased to the adequate control level when ω-3 FA–deficient mice were fed the ω-3 FA–adequate diet at either 3 wk or 4 mo of age.
FIGURE 4.
Effect of the ω-3 FA–adequate diet on hippocampal synaptic protein expression decreased in ω-3 FA–deficient mice. (A) Western blot results for hippocampal synaptic proteins from individual mice at 6 mo of age. (B) Relative protein expression normalized with GAPDH is expressed as means ± SEMs (n = 3). **P < 0.01, ***P < 0.001 (compared with the ω-3 FA–deficient group). Adeq, mice fed an ω-3 FA–adequate diet; Def, mice fed an ω-3 FA–deficient diet; Homer-1, Homer protein homolog 1; PSD-95, postsynaptic density protein 95; SNAP-25, synaptosome-associated protein of 25 kDa; Δ3 wk, diet changed at 3 wk of age; ∆4 mo, diet changed at 4 mo of age.
Discussion
In this study, we demonstrate that early correction of the brain DHA deficit is critical for preventing cognitive impairments in adult mice fed an ω-3 FA–deficient diet. Although brain DHA status can be normalized by ω-3 FA supplementation at any postnatal age, learning and memory function may be improved only when the offspring mice are fed an ω-3 FA–adequate diet immediately after weaning but not at a later stage.
Because mice are able to convert ALA to DHA with higher efficiency than humans, diets differing in ALA were used to manipulate DHA in the mouse brain. To deplete DHA in offspring brains, pregnant mice were fed a low-ALA (ω-3 FA–deficient) diet starting from gestational day 14 throughout the lactation period in our study. When offspring mice were fed a ω-3 FA–deficient diet, the brain DHA concentration at 6 mo was lowered to ∼50% of that of the Adeq group (Table 2). The DHA concentration was completely normalized when the ω-3 FA–deficient offspring were fed an ω-3 FA–adequate diet as late as 4 mo of age. The latter finding is consistent with results described earlier, in that only 8 wk of an ω-3 FA–adequate diet is sufficient for brain DHA to normalize in rats after dietary ω-3 FA depletion for 2 generations (17). Incomplete recovery of the DHA concentration in particular phospholipid classes (phosphatidylethanolamine and phosphatidylserine) after 24 wk of an ALA diet has also been reported in the rat hypothalamus (31). Our study indicates that ALA as a dietary ω-3 FA source is sufficient to normalize brain DHA in mice; however, in some cases, particularly in humans, DHA supplementation may be a more effective way to increase brain DHA concentration.
The functional deficits observed in ω-3 FA–deficient mice were remedied only if the deficient offspring were switched to the ω-3 FA–adequate diet at 3 wk of postnatal age (i.e., immediately after weaning), indicating that the perinatal period as a critical time for the development of DHA-dependent brain function. Our finding is consistent with previous reports showing that the lasting impact of ω-3 FA deficiency during the critical developmental time window is only incompletely rectified even after correcting the DHA deficit (19, 20, 32). However, Moriguchi and Salem (18) reported restoration of brain DHA levels and normalized cognitive behavior in the MWM task after feeding ω-3 FA–deficient young adult (7-wk-old) rats an ω-3 FA–adequate diet for 6 wk. This diet-reversal time point is close to 2 mo in our study for which significant impairment of learning and memory measured was still apparent at 6 mo of age even after 16-wk ω-3 FA replenishment (Figure 2C). The discrepancy may be attributable to an earlier diet-reversal time used in the previous work (at 7 wk) compared with our study (at 2 mo) or to different species used in these studies.
The finding that DHA deficiency must be corrected in the early perinatal period to normalize developmental function is also in agreement with the results reported for the visual system of rodents (33). De Velasco et al. (33) indicated that DHA supplementation early during postnatal life in ω-3 FA–deficient rats restored a normal pattern of connectivity of the retinocollicular pathway, whereas DHA supplementation after 4 wk of life did not improve visual development. A study by Kodas et al. (34) demonstrated the importance of the age at which ω-3 FA supplementation is initiated. In this study, tyramine-induced release of dopamine in adult rats (60-d-old) was ameliorated when ω-3 FA–deficient dams were placed on an ω-3 FA–adequate diet at or prior to 14 d gestation but not when this diet change occurred at 3 wk of age. These data, together with our results, similarly suggest the need for adequate ω-3 FA during development to prevent certain abnormalities in the adult.
Despite a very small sample size (n = 3), which is a potential limitation of this study, we observed changes in the hippocampal expression of synaptic proteins such as PSD-95 which is associated with learning and memory function (35–37), and is also affected by the brain DHA status (23, 24). Because synaptic proteins are essential for changes in synaptic plasticity and thus cognition, changes in their expression as a result of ω-3 FA deficiency and replenishment may be linked to changes in spatial learning and memory observed in response to these diets. The recovery of synaptic protein expression (Figure 4) and cognitive behavior (Figures 2 and 3) by a ω-3 FA diet was not observed when the diet was reversed at a later stage. However, it is possible that the duration of an ω-3 FA–adequate diet is also important and that animals that were deprived of ω-3 FA during development may still recover with regard to their neurochemical and cognitive responses if they are given an adequate diet for a longer duration. This alternate possibility cannot be ruled out, because the Δ3 wk group was fed the ω-3 FA–adequate diets longer than the Δ2 mo and Δ4 mo groups, making this a major limitation. This interpretation would lead to the conclusion that increasing brain DHA in an ω-3 FA–deficient state may eventually, but not immediately, result in improved biochemical and behavioral function.
In conclusion, this study demonstrates that an adequate supply of ω-3 FA during the perinatal period in mice is essential for supporting DHA-dependent cognitive function and hippocampal synaptic protein expression implicated in learning and memory. Learning and memory deficits in ω-3 FA–deficient offspring can be overcome only if dietary ω-3 FA supplementation is started early, although similar effects of supplementation for a much longer duration in the adult stage cannot be excluded. This finding underscores the importance of DHA administration for high-risk populations such as preterm infants in the immediate perinatal period, particularly when maternal ω-3 FA intake is suboptimal, as is often observed with modern dietary practices.
Acknowledgments
We thank Arthur Spector for reviewing the manuscript. The authors’ responsibilities were as follows—LEL and H-YK: designed the research, analyzed the data, had primary responsibility for the final content, and wrote the paper with contribution by coauthors; LEL and AD: conducted the animal experiments with statistical analysis; KK: performed the FA analysis; J-WL: performed the western blot analysis; and all authors: read and approved the final manuscript.
Footnotes
Abbreviations used: Adeq, mice fed an ω-3 fatty acid–adequate diet; ALA, α-linolenic acid; Def, mice fed an ω-3 fatty acid–deficient diet; DPAn-6, ω-6 docosapentaenoic acid; Homer-1, Homer protein homolog 1; PSD-95, postsynaptic density protein 95; SNAP-25, synaptosome-associated protein of 25 kDa; ∆ 2 mo, diet changed at 2 mo of age; Δ 3 wk, diet changed at 3 wk of age; ∆ 4 mo, diet changed at 4 mo of age.
References
- 1.Gharami K, Das M, Das S. Essential role of docosahexaenoic acid towards development of a smarter brain. Neurochem Int 2015;89:51–62. [DOI] [PubMed] [Google Scholar]
- 2.Crupi R, Marino A, Cuzzocrea S. n-3 Fatty acids: role in neurogenesis and neuroplasticity. Curr Med Chem 2013;20:2953–63. [DOI] [PubMed] [Google Scholar]
- 3.Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem 2004;90:979–88. [DOI] [PubMed] [Google Scholar]
- 4.Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, Kim HY. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem 2009;111:510–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HY, Moon HS, Cao D, Lee J, Kevala K, Jun SB, Lovinger DM, Akbar M, Huang BX. N-docosahexaenoylethanolamide promotes development of hippocampal neurons. Biochem J 2011;435:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rashid MA, Katakura M, Kharebava G, Kevala K, Kim HY. N-docosahexaenoylethanolamine is a potent neurogenic factor for neural stem cell differentiation. J Neurochem 2013;125:869–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hafandi A, Begg DP, Premaratna SD, Sinclair AJ, Jois M, Weisinger RS. Dietary repletion with omega 3 fatty acid or with COX inhibition reverses cognitive effects in F3 omega 3 fatty-acid deficient mice. Comp Med 2014;64:106–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Moriguchi T, Greiner RS, Salem N Jr. Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem 2000;75:2563–73. [DOI] [PubMed] [Google Scholar]
- 9.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev 1979;3:79–83. [DOI] [PubMed] [Google Scholar]
- 10.Ballabriga A, Martínez M. A chemical study on the development of the human forebrain and cerebellum during the brain ‘growth spurt’ period. II. Phosphoglyceride fatty acids. Brain Res 1978;159:363–70. [DOI] [PubMed] [Google Scholar]
- 11.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Hum Dev 1980;4:121–9. [DOI] [PubMed] [Google Scholar]
- 12.Sinclair AJ, Crawford MA. The accumulation of arachidonate and docosahexaenoate in the developing rat brain. J Neurochem 1972;19:1753–8. [DOI] [PubMed] [Google Scholar]
- 13.Kolb B, Whishaw IQ. Plasticity in the neocortex: mechanisms underlying recovery from early brain damage. Prog Neurobiol 1989;32:235–76. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson M. Formation of dendrites and development of synaptic connections. In: Developmental neurobiology. New York: Plenum Press; 1991. p. 223–284. [Google Scholar]
- 15.Makrides M, Neumann M, Simmer K, Gibson R, Pater J. Are long-chain polyunsaturated fatty acids essential nutrients in infancy? Lancet 1995;345:1463–8. [DOI] [PubMed] [Google Scholar]
- 16.Makrides M, Gibson RA, McPhee AJ, Collins CT, Davis PG, Doyle LW, Simmer K, Colditz PB, Morris S, Smithers LG, et al. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: a randomized controlled trial. JAMA 2009;301:175–82. [DOI] [PubMed] [Google Scholar]
- 17.Moriguchi T, Loewke J, Garrison M, Catalan JN, Salem N Jr. Reversal of docosahexaenoic acid deficiency in the rat brain, retina, liver, and serum. J Lipid Res 2001;42:419–27. [PubMed] [Google Scholar]
- 18.Moriguchi T, Salem N Jr. Recovery of brain docosahexaenoate leads to recovery of spatial task performance. J Neurochem 2003;87:297–309. [DOI] [PubMed] [Google Scholar]
- 19.Anderson GJ, Neuringer M, Lin DS, Connor WE. Can prenatal n-3 fatty acid deficiency be completely reversed after birth? Effects on retinal and brain biochemistry and visual function in rhesus monkeys. Pediatr Res 2005;58:865–72. [DOI] [PubMed] [Google Scholar]
- 20.Weisinger HS, Armitage JA, Sinclair AJ, Vingrys AJ, Burns PL, Weisinger RS. Perinatal omega-3 fatty acid deficiency affects blood pressure later in life. Nat Med 2001;7:258–9. [DOI] [PubMed] [Google Scholar]
- 21.McAllister AK. Dynamic aspects of CNS synapse formation. Annu Rev Neurosci 2007;30:425–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cansev M, Wurtman RJ, Sakamoto T, Ulus IH. Oral administration of circulating precursors for membrane phosphatides can promote the synthesis of new brain synapses. Alzheimers Dement 2008;4:s153–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidhu VK, Huang BX, Desai A, Kevala K, Kim HY. Role of DHA in aging-related changes in mouse brain synaptic plasma membrane proteome. Neurobiol Aging 2016;41:73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sidhu VK, Huang BX, Kim HY. Effects of docosahexaenoic acid on mouse brain synaptic plasma membrane proteome analyzed by mass spectrometry and (16)O/(18)O labeling. J Proteome Res 2011;10:5472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanguilder HD, Freeman WM. The hippocampal neuroproteome with aging and cognitive decline: past progress and future directions. Front Aging Neurosci 2011;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saura CA, Parra-Damas A, Enriquez-Barreto L. Gene expression parallels synaptic excitability and plasticity changes in Alzheimer’s disease. Front Cell Neurosci 2015;9:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bligh EG, Dyer WJ. A rapid method for total lipid extraction and purification. Can J Biochem Physiol. 1959;7:911–17. [DOI] [PubMed] [Google Scholar]
- 28.Wen Z, Kim HY. Alterations in hippocampal phospholipid profile by prenatal exposure to ethanol. J Neurochem 2004;89:1368–77. [DOI] [PubMed] [Google Scholar]
- 29.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 1984;11:47–60. [DOI] [PubMed] [Google Scholar]
- 30.Lee JW, Huang BX, Kwon H, Rashid MA, Kharebava G, Desai A, Patnaik S, Marugan J, Kim HY. Orphan GPR110 (ADGRF1) targeted by N-docosahexaenoylethanolamine in development of neurons and cognitive function. Nat Commun 2016;7:13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li D, Weisinger HS, Weisinger RS, Mathai M, Armitage JA, Vingrys AJ, Sinclair AJ. Omega 6 to omega 3 fatty acid imbalance early in life leads to persistent reductions in DHA levels in glycerophospholipids in rat hypothalamus even after long-term omega 3 fatty acid repletion. Prostaglandins Leukot Essent Fatty Acids 2006;74:391–9. [DOI] [PubMed] [Google Scholar]
- 32.Weiser MJ, Wynalda K, Salem N Jr., Butt CM. Dietary DHA during development affects depression-like behaviors and biomarkers that emerge after puberty in adolescent rats. J Lipid Res 2015;56:151–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Velasco PC, Sandre PC, Tavares Do Carmo MG, Faria-Melibeu AC, Campello-Costa P, Ferraz AC, Andrade Da Costa BL, Serfaty CA. A critical period for omega-3 nutritional supplementation in the development of the rodent visual system. Brain Res 2015;1615:106–15. [DOI] [PubMed] [Google Scholar]
- 34.Kodas E, Vancassel S, Lejeune B, Guilloteau D, Chalon S. Reversibility of n-3 fatty acid deficiency-induced changes in dopaminergic neurotransmission in rats critical role of developmental stage. J Lipid Res 2002;43:1209–19. [PubMed] [Google Scholar]
- 35.Chen G, Hu T, Li Q, Li JK, Jia Y, Wang Z. Expression of synaptosomal-associated protein-25 in the rat brain after subarachnoid hemorrhage. Neural Regen Res 2013;8:2693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gandhi RM, Kogan CS, Messier C, MacLeod LS. Visual-spatial learning impairments are associated with hippocampal PSD-95 protein dysregulation in a mouse model of fragile X syndrome. Neuroreport 2014;25:255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerstein H, Lindstrom MJ, Burger C. Gene delivery of Homer1c rescues spatial learning in a rodent model of cognitive aging. Neurobiol Aging 2013;34:1963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]