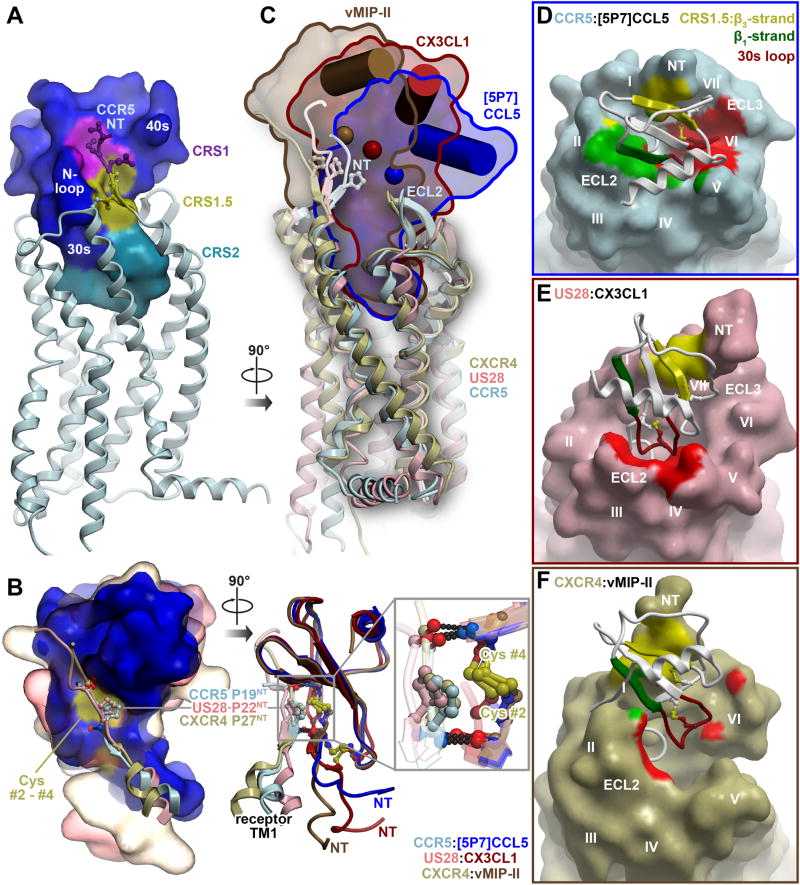
Figure 2. The CCR5-[5P7]CCL5 complex shows additional, distinct interaction epitopes in the context of the conserved two-site architecture.
(A) Three epitopes observed in earlier receptor-chemokine structures are highlighted on the structure of CCR5-[5P7]CCL5: CRS1 (purple), CRS1.5 (gold), and CRS2 (cyan).
(B) Comparison of the CRS1.5 interaction geometry and hydrogen bonding pattern between CCR5-[5P7]CCL5 and earlier receptor-chemokine structures. Chemokines are superimposed by their globular cores and shown as molecular surfaces on the left and ribbons on the right. The fragments involving the N-terminus and helix I of each receptor are shown as ribbons with the conserved Pro of the 19-PC-20 motif in sticks. The Pro invariably packs against the conserved disulfide (yellow surface on left and yellow sticks on right) of the chemokine, and the flanking residues form invariable backbone hydrogen bonds to the chemokine proximal N-terminus and β3-strand.
(C) The diverse positions of the chemokine globular cores with respect to the TM domains of the receptors are shown for CCR5-[5P7]CCL5, US28-CX3CL1 and CXCR4-vMIP-II. The TM domains are superimposed and shown in ribbons, chemokines are shown as molecular surfaces and contoured for clarity. The C-terminal helix and Cα atom of the central residue of the β-sheet (V40 in CCL5) of each chemokine are shown as a cylinder and a sphere, respectively.
(D-F) The interactions formed by the [5P7]CCL5 β1-strand with ECL2 of CCR5 (green) and its 30s loop with CCR5 ECL3 and helices V-VII deep within the CCR5 binding pocket (red), are highlighted and compared with similar regions of US28-CX3CL1 and CXCR4-vMIP-II in (E and F). The β1-strand interaction is virtually absent from both US28-CX3CL1 (E) and CXCR4-vMIPII (F) complexes. The chemokine 30s loop contacts ECL2 in the US28-CX3CL1 complex (E) but shows almost no interaction in the CXCR4-vMIPII complex (F).
See also Figure S2.