Abstract
Background
Fms-like tyrosine kinase 3-internal tandem duplication (FLT3-ITD) mutations are common in acute myeloid leukemia (AML) and are associated with rapid relapse and short survival. In relapsed/refractory (R/R) AML, the clinical benefit of FLT3 inhibitors has been limited by rapid generation of resistance mutations, especially FLT3-D835. Gilteritinib is a potent, highly selective oral FLT3/AXL inhibitor with preclinical activity against FLT3-ITD and FLT3-D835 mutations. The aim of this Phase 1/2 study was to assess the safety, tolerability, and pharmacokinetic (PK) effects of gilteritinib in FLT3 mutation-positive (FLT3mut+) R/R AML.
Methods
This ongoing pharmacodynamic-driven Phase 1/2 trial (NCT02014558) enrolled subjects from October 2013 to August 2015 who were aged ≥18 years and were either refractory to induction therapy or had relapsed after achieving remission with prior therapy. Subjects were enrolled in one of seven dose-escalation or dose-expansion cohorts that were assigned to receive once-daily doses of oral gilteritinib (20, 40, 80, 120, 200, 300, or 450 mg). Cohort expansion was based on safety/tolerability, FLT3 inhibition in correlative assays, and antileukemic activity; the 120 and 200 mg dose cohorts were further expanded to include FLT3mut+ patients only. Safety and tolerability, and PK effects were the primary endpoints; antileukemic response was the main secondary endpoint. Safety and tolerability were assessed by monitoring dose-limiting toxicities and treatment-emergent adverse events, and safety assessments (eg, clinical laboratory evaluations, electrocardiograms) in the Safety Analysis Set.
Findings
A total of 252 adults with R/R AML, including 58 with wild-type FLT3 and 194 with FLT3 mutations (FLT3-ITD, n=162; FLT3-D835, n=16; FLT3-ITD and -D835, n=13; other, n=3), received oral gilteritinib (20–450 mg) once daily in one of seven dose-escalation (n=23) or dose-expansion (n=229) cohorts. Gilteritinib was well tolerated in this heavily pretreated population; Grade 3 diarrhea and hepatic transaminase elevation limited dosing above 300 mg/d. The most common Grade 3/4 adverse events were febrile neutropenia (39%; n=97/252), anemia (24%; n=61/252), thromobocytopenia (13%; n=33/252), sepsis (11%; n=28/252), and pneumonia (11%; n=27/252). Serious adverse events in ≥5% of patients were febrile neutropenia (31%; n=78/252), progressive disease (17%; n=43/252), sepsis (14%; n=36/252), pneumonia (11%; n=27/252), and acute renal failure (10%; n=25/252), pyrexia (8%; n=21/252), bacteremia (6%; n=14/252), and respiratory failure (6%; n=14/252). Gilteritinib demonstrated consistent, potent inhibition of FLT3 phosphorylation at doses ≥80 mg/d in correlative assays. While responses were observed across all dose levels regardless of FLT3 mutation status (overall response rate [ORR]=40%), response rate was improved in FLT3mut+ patients at doses ≥80 mg/d (ORR=52%). Among patients with FLT3-ITD, the additional presence of FLT3-D835 did not alter response rate; patients with only FLT3-D835 responded less frequently.
Interpretation
Gilteritinib had a favorable safety profile and generated potent FLT3 inhibition leading to high rates of antileukemic responses in patients with FLT3mut+ R/R AML. These findings confirm that FLT3 is a high-value target in R/R AML and that long-term success of therapeutic FLT3 inhibition in AML is optimized by agents with potent, selective, and sustained activity against FLT3-ITD mutations and FLT3 tyrosine kinase domain mutations.
Funding
This study was funded by Astellas Pharma, Inc., by a National Cancer Institute Leukemia Specialized Program of Research Excellence grant (CA100632) awarded to Drs Mark Levis and Jorge Cortes, and by Associazione Italiana Ricerca sul Cancro awarded to Professor Giovanni Martinelli.
Keywords: FLT3 inhibition, acute myeloid leukemia, relapsed/refractory, tyrosine kinase inhibitor
INTRODUCTION
Fms-like tyrosine kinase 3 (FLT3) is one of the most frequently mutated genes in acute myeloid leukemia (AML), with mutations occurring in up to 30% of patients.1–3 Two distinct activating FLT3 mutations occur at different hotspots: internal tandem duplications in the juxtamembrane domain (FLT3-ITD)4 and point mutations in the tyrosine kinase domain (FLT3-TKD), most commonly at codon D835.5 Clinically, FLT3 mutations are associated with an aggressive disease course; FLT3-ITD mutations are strong predictors of rapid relapse and short overall survival (OS) after chemotherapy.2
Development of drugs that effectively target mutated FLT3 has been challenging. Initial FLT3 inhibitors had poor bioavailability, limited potency, inadequate kinase specificity, low response rates, and short response duration.6–9 Although some FLT3 inhibitors, including the multi-kinase inhibitor sorafenib, generated significant antileukemic effects,10,11 response rates were variable and typically transient in the FLT3-ITD AML population.10,12–14 Furthermore, treatment resistance generally emerged within a few weeks of treatment initiation.15–17 One mechanism of treatment resistance is the development of secondary FLT3-TKD mutations.15–17 As such, gilteritinib (ASP2215), an oral FLT3/AXL inhibitor, was designed to specifically target patients with FLT3-ITD mutations and address the limitations of other FLT3-targeted therapies.
In vitro, gilteritinib has shown selective kinase inhibition of FLT3 and highly potent activity against FLT3 receptors with ITD and TKD mutations.18 Gilteritinib has demonstrated antileukemic activity in cell lines expressing FLT3-D835 mutations, suggesting it may be effective against the most commonly acquired point mutation conferring resistance to other FLT3 inhibitors.18 Moreover, gilteritinib inhibits AXL,18 an oncogenic tyrosine kinase frequently overexpressed in AML19 that facilitates FLT3 activation and has been implicated in FLT3 inhibitor resistance.20,21 An investigation of the inhibitory effects of gilteritinib against 78 different kinases demonstrated that gilteritinib was a strong inhibitor of FLT3 and AXL as well as anaplastic lymphoma kinase (ALK), and leukocyte receptor tyrosine kinase (LTK).22 In cell-based assays the IC50 was 1–2 nM for FLT3 and 102 nM for c-Kit.18 In vitro assays also demonstrated that gilteritinib had weaker activity against FLT3-F691 gatekeeper mutations requiring higher concentrations to achieve IC50 than those observed for FLT3-ITD and FLT3-D835.18 Based on these preclinical findings, a pharmacodynamic-driven, first-in-human phase 1/2 trial was conducted in adult patients with relapsed/refractory (R/R) AML (NCT02014558). The primary objectives were to assess the safety and tolerability of gilteritinib and to determine the maximum tolerated dose (MTD). Additionally, we sought to define the optimal phase 3 dose, based on clinical response and in vivo FLT3 inhibition, and establish the pharmacokinetic (PK) profile of gilteritinib. A key secondary objective was to assess the antileukemic activity in FLT3-mutation positive (FLT3mut+) and wild-type FLT3 (FLT3WT) AML patient populations.
METHODS
Study Design and Participants
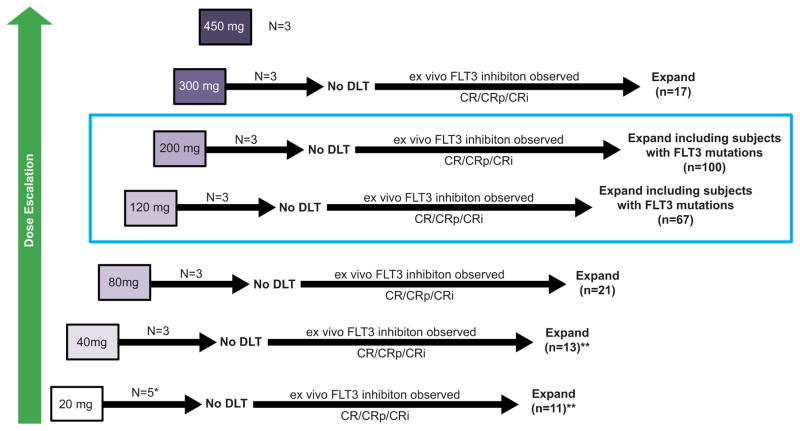
This international, open-label, Phase 1/2, dose-escalation/dose-expansion study was conducted from October 2013 through November 2015 across 28 sites in the United States, France, Germany, and Italy. The database was locked for final analysis in November 2015 and data cleaning was completed by May 2016. The study had seven dose-escalation cohorts (20–450 mg) with ≥3 patients enrolled at each dose level (Figure 1). The decision to proceed to the next dose cohort was made by the dose-escalation committee (Supplemental Appendix, page 1) and followed an accelerated titration design. Dose escalation continued until ≥2 dose-limiting toxicities (DLTs) were observed in a cohort of three to six patients; when ≥2 DLTs occurred in a dose level, the next lower dose level was declared the MTD. Safety in all dose cohorts was monitored with respect to DLTs using the Bayesian continual reassessment method and the posterior DLTs mean was calculated to confirm the MTD that was determined from the dose-escalation cohorts.
Figure 1. Study Design and Accrual.
* Three evaluable subjects
** Enrollment stopped early for low response rate
CR, complete remission; CRi, complete remission with incomplete hematologic recovery; CRp, complete remission with incomplete platelet recovery; DLT, dose-limiting toxicity; FLT3, Fms-like tyrosine kinase 3
Following escalation to the next dose cohort, additional patients were enrolled to the dose-expansion cohorts if the median decrease in FLT3 phosphorylation was ≥90% as determined by an ex vivo FLT3 plasma inhibitory activity (PIA) assay23 or if ≥1 subject achieved a complete remission (CR), CR with incomplete hematological recovery (CRi), or CR with incomplete platelet recovery (CRp; Table S1, Supplemental Appendix, page 3). If FLT3 target inhibition was observed or CR/CRi/CRp occurred, and no DLTs were observed in the initial three patients, dose cohorts were expanded to include ≥14 additional patients. On the basis of emerging toxicity, pharmacokinetic/pharmacodynamic profile, and antileukemic response, the 120 and 200 mg dose cohorts were further expanded to include FLT3mut+ patients only.
Patients (≥18 years) with primary or secondary AML and Eastern Cooperative Oncology Group (ECOG) performance status of ≤2 were eligible for enrollment if they were refractory to ≥1 cycle of induction chemotherapy or had relapsed after achieving remission with a prior therapy. Although the presence of a FLT3 mutation was not an inclusion criterion, ≥10 patients with locally confirmed FLT3 mutations (ITD or activating point mutation) were required to be enrolled in the expansion cohorts at each dose level. Other inclusion criteria were serum alanine or aspartate aminotransferase levels ≤2.5 × the institutional upper limit of normal (ULN), serum creatinine levels ≤1.5 × institutional ULN or an estimated glomerular filtration rate >50 ml/min. Patients who had congestive heart failure New York Heart Association (NYHA) class 3/4 were excluded; those who previously had NYHA class 3/4 congestive heart failure were excluded, unless a screening echocardiogram performed within 3 months prior to study entry resulted in a left ventricular ejection fraction ≥45%. Patients with long QTc syndrome at screening and those with active uncontrolled infections, including hepatitis B or C infections and human immunodeficiency virus infection, were also excluded. Additional inclusion/exclusion criteria are detailed in the Supplemental Appendix (page 1).
Procedures
The first patient was treated at a starting dose of 20 mg/d; if no DLT occurred, the next patient was enrolled at the next dose level of 40 mg/d. Dose escalation ≤80 mg/d continued until the first instance of a DLT or second instance of a grade 2 AE judged by the investigator to be related to the study drug with the exception of hematologic toxicities. At doses >80 mg/d, three subjects were treated at each subsequent dose level (120 mg/d, 200 mg/d, 300 mg/d, and 450 mg/d), such that if no DLTs were observed at a specific dose level, the subsequent three patients were treated at the next dose level. If a DLT was observed in one of three subjects at a particular dose level, three additional patients were enrolled at the same dose level; if two or more DLTs were observed at a specific dose level, DLT will be established and the next lower dose level will be established as the MTD. For the dose-expansion cohorts, ≥10 patients with FLT3-ITD mutations were to be included at each dose level, and dose levels ≥120 mg/d were further expanded to include ≥42 FLT3mut+ patients on the basis of antileukemic response.
Patients received an initial oral gilteritinib dose (tablet) followed by a 48-hour observation and PK analysis period (Cycle 0). Starting on Day 1 Cycle 1, patients entered a repeated-dose protocol and received once-daily oral gilteritinib in a 28-day cycle that continued uninterrupted in the absence of significant toxicity; all subsequent cycles followed this repeated-dose protocol, with patients continuing on protocol until a discontinuation criterion was met. In the original protocol, patients who achieved CR/CRp/CRi or partial remission (PR) were permitted to undergo hematopoietic stem cell transplantation (HSCT) after stopping gilteritinib treatment; however, a protocol amendment issued after enrollment had started allowed patients to re-enroll after HSCT and resume gilteritinib treatment (Supplemental Appendix, pages 1–2).
Dose interruptions were specified in cases of grade 2 retinopathy, and grade 3 non-hematologic AEs that were at least possibly related to gilteritinib. After resolution of these toxicities, gilteritinib could be restarted at a lower dose. Dose reduction without interruption was also allowed for persistent myelosuppression after two cycles of therapy in patients who had <5% bone marrow blasts and no extramedullary disease. Treatment discontinuations were specified for grade 3/4 retinopathy, any grade 4 non-hematologic toxicity that was at least possibly related to the study drug. For patients in the dose escalation cohort who received 20 to 40 mg/d gilteritinib, a single dose increase was permitted if CRc had not been attained after Cycle 1; dose increases were not permitted for other dose escalation cohorts. For patients in the dose expansion cohort, stepwise dose escalation was allowed if CRc had not been attained after Cycle 1.
Discontinuation criteria during the course of the study were withdrawal of consent, significant deviation from specified inclusion or exclusion criteria (subjects having a clinical benefit and no DLT could remain in the study after discussion with the medical monitor), no response (ie, no partial or composite remission) to treatment and according to the investigator’s opinion no longer deriving clinical benefit after 2 cycles of treatment; gilteritinib dose interruption for >15 days (subjects may resume treatment after discussion with the medical monitor if the dose interruption did not stem from a treatment-related adverse event [AE]); determination by the investigator that continuation of the study drug will be detrimental to the patient, loss to follow-up, and death. Posttreatment discontinuation criteria were withdrawal of consent, loss to follow-up, lapse of >3 years from the End of Treatment visit, or death.
Safety and tolerability were assessed by the study investigators. Both TEAEs and clinical laboratory evaluations were scheduled at screening, on Days −2 and −1 before Cycle 1; during Cycle 1 on Days 1, 4±1, 8±1, 15, and 22±1; during Cycle 2 on Days 1±3 and 15±1; during subsequent cycles on Day 1±3; and at the end-of-treatment visit (within 7 days of the last dose of the study drug). Assessment of TEAEs also occurred at the 30-day follow-up visit. Full details regarding clinical safety/tolerability as well as PK and PD assessments are presented in the Supplemental Appendix (page 2). The antileukemic response following treatment with gilteritinib was determined using modified International Working Group (IWG) criteria.24,12 Bone marrow aspirates and biopsies were obtained at the time of study entry and subsequently on the first day of Cycles 2 and 3. Bone marrow aspirate and biopsies were repeated at one month after achievement of first CRc to confirm response and every 3 cycles thereafter. All other patients underwent bone marrow assessments every 2 cycles.
Outcomes
Primary endpoints were safety, tolerability, and PK profile of gilteritinib; secondary endpoints were the antileukemic effects of gilteritinib and evaluation of gilteritinib drug-drug interactions (DDI). Exploratory endpoints were the antitumor activity of gilteritinib with respect to FLT3, C-CBL, and AXL mutations, effects of gilteritinib on biomarkers related to AML, relationship between the PK profile and the pharmacodynamic (PD) effects of gilteritinib, evaluation of AXL mutations and AXL expression, PIA, PD effects of gilteritinib with respect to FLT3, AXL, and S6 phosphorylation. Gilteritinib DDI and many of the exploratory endpoints findings are to be addressed in separate publications. Composite remission (CRc) rate was defined as the sum of patients achieving CR, CRi, and CRp. Overall response rate (ORR) was defined as the sum of patients achieving CRc and PR. The duration of response (DOR) was defined as the time from the date of either first CRc or PR until the date of documented relapse of any time for subjects who achieve CRc or PR (relapse date – first CRc or PR disease assessment date + 1). Subjects who died without report of relapse were considered non-events and censored at their last relapse-free disease assessment date (last relapse-free assessment date – first CRc or PR disease assessment date + 1). Subjects who discontinued the study for an allogeneic HSCT were considered non-events and were censored at the time of HSCT. Other subjects who did not relapse during the study were considered non-events and were censored at the last relapse-free assessment date.
Tolerability and safety were based on local site-specific monitoring of DLTs and treatment-emergent adverse events (TEAEs), as well as on physical examinations, clinical laboratory evaluations, and electrocardiogram (ECG) monitoring. A DLT was defined as an event that was considered possibly or probably related to treatment, which occurred within 30 days of the starting dose on Day −2 for the dose-escalation cohorts or during the 28-day cycle for the expansion cohorts. DLTs were any Grade ≥3 non-hematologic or extramedullary toxicity with the exception of anemia, anorexia, fatigue, Grade 3 infection, Grade 3 fever with neutropenia, Grade 3 diarrhea or nausea that did not require tube-feeding or total parenteral nutrition, or diarrhea that could be managed to Grade ≤2 with standard medications. Of note, hematological toxicities were not considered DLTs.
Statistical Analysis
Sample size was not based on a statistical power calculation but rather on study design. It was estimated that upwards of 270 patients would be enrolled and that this sample size would provide adequate information for the study objectives.
Safety endpoints were analyzed in the Safety Analysis Set (SAF), defined as all patients who took ≥1 dose of gilteritinib. A two-parameter Bayesian logistical regression model was used to determine the dose-toxicity relationship and to inform the determination of MTD.25 All AEs and clinical laboratory data were summarized using descriptive statistics. Pharmacokinetic effects were assessed in the Pharmacokinetic Analysis Set, which is a subset of the SAF for which sufficient plasma concentration data were available to enable derivation of ≥1 PK parameter. Blood samples were drawn from patients to assess PK and PD parameters. PK parameters were evaluated by cohort and reported using descriptive statistics; analysis of PD effects using PIA has been described previously.23 Antileukemic activity was assessed in the Full Analysis Set (FAS), defined as all enrolled patients who received ≥1 dose of drug and had ≥1 post-treatment data point. Remission rates, CRc rates, ORR, duration of confirmed response, and overall survival (OS) were summarized using descriptive statistics. The OS curves and median time-to-event variables were estimated using the Kaplan–Meier method. To explore the relationship between dose level and CR response, a dose-response model was fitted to the binary CR response with FLT3 mutation status and the first and second order of logarithm-transformed dose, as independent covariates for all patients from the dose-escalation and dose-expansion cohorts. The CR rate with two-sided 95% confidence interval (CI) for each dose level was estimated from this model. All statistical analyses were performed using SAS® (Version 9·3 or higher) software.
STUDY OVERSIGHT
This study was designed by the study sponsor in collaboration with the investigators, and was conducted in accordance with Declaration of Helsinki ethical principles, Good Clinical Practices, principles of informed consent, and requirements of public registration of clinical trials (ClinicalTrials.gov Identifier, NCT02014558). Site-specific institutional review boards approved the protocol. Written informed consent was obtained from each subject at enrollment. Statistical analyses were performed by a statistician at Astellas Pharma, Inc. All authors were in agreement regarding submission of the manuscript and vouch for the completeness and accuracy of the data. Professional medical writers, paid for by the study sponsor, assisted with manuscript preparation and submission under the authors’ guidance.
ROLE OF THE FUNDING SOURCE
Astellas Pharma, Inc. provided funding for this trial and was involved in the development of the study protocol, and in data collection, analysis, and interpretation. Editorial and writing assistance during the development of the manuscript was supported by the funder. The corresponding author had full access to all of the data in the study and final responsibility for the decision to submit the paper for publication.
RESULTS
STUDY DISPOSITION
The first subject in this study was enrolled on October 15, 2013; the last patient was enrolled on August 27, 2015. In total, 265 patients were allocated to treatment at 28 sites across the United States and Europe (Table S2, Supplemental Appendix, page 4). As of November 2015, 234 (88%) had discontinued treatment; 31 (12%) patients remained on treatment with a median treatment duration of 25·9 weeks (interquartile range [IQR]: 15–50). The SAF included 252 patients (n=23, dose escalation; n=229, dose expansion) enrolled across seven dose cohorts (20–450 mg); 13 of the 265 enrolled patients were excluded from the SAF because they did not receive treatment with the study drug. The FAS consisted of 249 patients and the Pharmacokinetic Analysis Set comprised 19 patients. Demographics and baseline characteristics of patients in the SAF are presented in Table 1. FLT3-ITD mutation at screening was locally confirmed in 162 patients, FLT3-D835 in 13 patients, and dual FLT3-ITD-D835 in 16 patients; all 31 patients remaining on treatment at database lock carried a FLT3-ITD mutation. All patients in the SAF had received prior chemotherapy, 29% (n=73/252) had undergone prior HSCT, and 25% (n=63/252) had received a prior tyrosine kinase inhibitor (TKI), most commonly sorafenib (n=54; Table 1). Among patients who had received prior TKI therapy, 3 (1%) had received ≥2 TKIs; all three subjects received lower doses of gilteritinib (20 mg, n=1; 40 mg, n=2).
Table 1.
Baseline Demographics and Disease Characteristics (SAF)
| 20 mg (n=16) | 40 mg (n=16) | 80 mg (n=24) | 120 mg (n=70) | 200 mg (n=103) | 300 mg (n=20) | 450 mg (n=3) | |
|---|---|---|---|---|---|---|---|
| Age (years), median (IQR) | 64·5 (58, 71) | 62 (54, 66 ) | 62 (47, 70) | 59·5 (51, 69) | 64 (49, 70) | 64 (46, 69) | 64 (50, 71) |
| Sex, n | |||||||
| Male | 6 (38%) | 11 (69%) | 11 (46%) | 32 (46%) | 52 (51%) | 14 (70%) | 3 (100%) |
| Female | 10 (63%) | 5 (31%) | 13 (54%) | 38 (54%) | 51 (50%) | 6 (30%) | 0 |
| Cytogenetic risk status, na | |||||||
| Favorable | 0 | 0 | 2 (8%) | 1 (1%) | 3 (3%) | 0 | 1 (33%) |
| Intermediate | 13 (81%) | 5 (31%) | 11 (46%) | 42 (60%) | 64 (62%) | 8 (40%) | 0 |
| Unfavorable | 2 (13%) | 9 (56%) | 7 (29%) | 12 (17%) | 17 (17%) | 7 (35%) | 2 (67%) |
| AML disease history | |||||||
| Duration of disease (months), median (IQR) | 10·6 (7·2, 16·1) | 7·1 (5·1, 11·7) | 16·8 (8·3, 29) | 9·0 (4·7, 16·6) | 8·3 (3·9, 13·8) | 7·3 (2·7, 16·5) | 6·3 (3·5, 11·9) |
| Prior stem cell transplant, n | |||||||
| 0 | 11 (69%) | 13 (81%) | 15 (63%) | 49 (70%) | 71 (69%) | 18 (90%) | 2 (67%) |
| 1 | 4 (25%) | 2 (13%) | 9 (38%) | 20 (29%) | 29 (28%) | 2 (10%) | 1 (33%) |
| ≥2 | 1 (6%) | 1 (6%) | 0 | 1 (1%) | 3 (3%) | 0 | 0 |
| Lines of prior AML therapy, n | |||||||
| 1 | 3 (19%) | 5 (31%) | 5 (21%) | 17 (24%) | 36 (35%) | 7 (35%) | 2 (67%) |
| 2 | 3 (19%) | 1 (6%) | 5 (21%) | 22 (31%) | 28 (27%) | 7 (35%) | 0 |
| ≥3 | 10 (63%) | 10 (63%) | 14 (58%) | 31 (44%) | 39 (38%) | 6 (30%) | 1 (33%) |
| Prior TKI therapy, n | |||||||
| No | 8 (50%) | 12 (75%) | 19 (79%) | 48 (69%) | 82 (80%) | 18 (90%) | 2 (67%) |
| Yes | 8 (50%) | 4 (25%) | 5 (21%) | 22 (31%) | 21 (20%) | 2 (10%) | 1 (33%) |
| TKI therapy usagea,b, n | |||||||
| Sorafenib | 6 (75%) | 4 (100%) | 5 (100%) | 19 (83%) | 18 (82%) | 1 (50%) | 1 (100%) |
| PLX 3397 | 2 (25%) | 2 (50%) | 1 (20%) | 3 (13%) | 0 | 0 | 0 |
| Quizartinib | 0 | 0 | 0 | 1 (4%) | 4 (18%) | 1 (50%) | 0 |
| Crenolanib | 1 (13%) | 0 | 0 | 1 (4%) | 0 | 0 | 0 |
| FLT3 mutation type, n c | |||||||
| FLT3-ITD | 12 (75%) | 6 (38%) | 10 (42%) | 47 (67%) | 79 (77%) | 8 (40%) | 0 |
| FLT3-D835 | 1 (6%) | 0 | 1 (4%) | 6 (9%) | 3 (3%) | 1 (5%) | 1 (33%) |
| FLT3-ITD and -D835 | 1 (6%) | 2 (13%) | 1 (4%) | 3 (4%) | 8 (8%) | 0 | 1 (33%) |
AML, acute myeloid leukemia; FLT3, FMS-like tyrosine kinase; IQR, interquartile range; SAF, safety analysis set; TKI, tyrosine kinase inhibitor.
Values represent the proportion of patients who had received prior TKI therapy
Patients may have received more than one prior TKI;
FLT3 mutation status was determined by a local laboratory.
SAFETY AND TOLERABILITY PROFILE OF GILTERITINIB (20–450mg)
Treatment-emergent AEs occurring in ≥10% of the SAF by grade is provided in Table 2; a list of any TEAE of Grade ≥3 occurring in <10% of the SAF is provided in Table S3 (Supplemental Appendix, pages 5–12). Commonly reported AEs considered related to gilteritinib were typical for AML therapies (diarrhea [16%; n=41/252], fatigue [15%; n=37/252], elevated AST [13%; [n=33/252], elevated ALT [10%; n=24/252]). Dose reductions were required for 10% (n=25/252) of patients in the SAF. Additional data regarding on-study exposure to gilteritinib can be found in Table S4 (Supplemental Appendix, page 13). Adverse events commonly associated with dose reductions were diarrhea (1%; n=2/252) and fatigue (1%; n=3/252); serious AEs observed in ≥5% of SAF patients were febrile neutropenia (31%; n=78/252), progressive disease (17%; n=43/252), sepsis (14%; n=36/252), pneumonia (11%; n=27/252), acute renal failure (10%; n=25/252), pyrexia (8%; n=21/252), bacteremia (6%; n=14/252), and respiratory failure (6%; n=14/252). Of these serious AEs, febrile neutropenia (2%; n=5/252), acute renal failure (2%; n=5/252), pyrexia (1%; n=3/252), sepsis (1%; n=2/252), and bacteremia (0.4%; n=1/252) were considered related to treatment. Eleven of 252 patients (4%) had a maximum post-baseline QTcF interval >500 ms, and 22 of 252 patients (9%) had a >60 ms change in their maximum post-baseline QTcF relative to baseline. One critically ill patient in the 120 mg dose cohort, with no cardiac comorbidities, experienced fatal ventricular fibrillation deemed possibly related to treatment. Of the 37 patients who underwent allogeneic transplantation, 13 resumed gilteritinib treatment after HSCT; three of these patients died due to causes unrelated to gilteritinib.
Table 2.
Incidence of Treatment-Emergent Adverse Events*
| Grade 1/2 Treatment-Emergent Adverse Events Occurring in ≥10% of Patients (N=252)# | Grade ≥3 Treatment-Emergent Adverse Events Occurring in ≥2% of Patients (N=252) | ||||
|---|---|---|---|---|---|
| Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) | Grade 4, n (%) | Grade 5, n (%) | |
| Febrile neutropenia | 0 | 0 | 9 (36%) | 7 (3%) | 1 (0·4%) |
| Diarrheaa,b | 47 (19%) | 32 (13%) | 13 (5%) | 0 | 0 |
| Anemia | 1 (0·4%) | 23 (9%) | 55 (22%) | 6 (2%) | 1 (0·4%) |
| Fatigue | 31 (12%) | 37 (15%) | 15 (6%) | 0 | 0 |
| Edema peripheral | 43 (17%) | 21 (8%) | 3 (1.2%) | 0 | 0 |
| Elevated ASTa,b | 33 (13%) | 18 (7%) | 14 (6%) | 1 (0·4%) | 0 |
| Pyrexiaa | 36 (14%) | 16 (6%) | 13 (5%) | 0 | 0 |
| Dyspnea | 29 (12%) | 18 (7%) | 11 (4%) | 1 (0.4%) | 0 |
| Constipation | 40 (16%) | 17 (7%) | 0 | 0 | 0 |
| Cough | 40 (16%) | 14 (6%) | 0 | 0 | 0 |
| Nausea | 30 (12%) | 19 (8%) | 5 (2%) | 0 | 0 |
| Dizziness | 36 (14%) | 11 (4%) | 0 | 0 | 0 |
| Epistaxis | 36 (14%) | 9 (4%) | 0 | 0 | 0 |
| Elevated ALT | 25 (10%) | 9 (4%) | 13 (5%) | 0 | 0 |
| Vomiting | 35 (14%) | 7 (3%) | 5 (2%) | 0 | 0 |
| Hypotension | 7 (3%) | 21 (8%) | 16 (6%) | 2 (0·8%) | 0 |
| Hypokalaemia | 22 (9%) | 14 (6%) | 7 (3%) | 2 (0·8%) | 0 |
| Progressive AML | 0 | 0 | 2 (0.8) | 0 | 41 (16%) |
| Hypocalcaemia | 13 (5%) | 15 (6%) | 14 (6%) | 1 (0·4%) | 0 |
| Decreased platelet count | 1 (0·4%) | 3 (1·2%) | 7 (3%) | 30 (12%) | 0 |
| Elevated blood creatinine | 23 (9%) | 12 (5%) | 0 | 0 | 0 |
| Thrombocytopenia | 1 (0·4%) | 5 (2%) | 2 (0·8%) | 31 (12%) | 0 |
| Sepsis | 0 | 1 (0·4%) | 5 (2%) | 23 (9%) | 7 (3%) |
| Fall | 17 (7%) | 10 (4%) | 7 (3%) | 1 (0.4) | 0 |
| Pneumonia | 1 (0·4%) | 3 (1·2%) | 26 (10%) | 1 (0·4%) | 4 (1·6%) |
| Hyponatraemia | 18 (7%) | 4 (1·6%) | 10 (4%) | 2 (0·8%) | 0 |
| Elevated blood alkaline phosphatase | 28 (11%) | 5 (2%) | 0 | 0 | 0 |
| Hypomagnesemia | 29 (12%) | 2 (0·8%) | 0 | 0 | 0 |
| Decreased appetite | 12 (5%) | 18 (7%) | 0 | 0 | 0. |
| Hypoalbuminemia | 5 (2%) | 22 (9%) | 5 (2%) | 0 | 0 |
| Headache | 22 (9%) | 6 (2%) | 0 | 0 | 0 |
| Asthenia | 14 (6%) | 10 (4%) | 6 (2%) | 0 | 0 |
| Arthralgia | 19 (8%) | 8 (3%) | 0 | 0 | 0 |
| Hypoxia | 1 (0·4%) | 8 (3%) | 13 (5%) | 5(2%) | 1 (0·4%) |
| Dysgeusia | 23 (9%) | 4 (1·6%) | 0 | 0 | 0 |
| Stomatitis | 12 (5%) | 10 (4%) | 0 | 0 | 0 |
| Decreased neutrophil count | 0 | 5 (2%) | 7 (3%) | 14 (6%) | 0 |
| Insomnia | 14 (6%) | 10 (4%) | 0 | 0 | 0 |
| Acute renal failure | 7 (3%) | 9 (4%) | 8 (3%) | 1 (0·4%) | 0 |
| Hypertension | 6 (2%) | 11 (4%) | 8 (3%) | 0 | 0 |
| Neutropenia | 0 | 2 (0·8%) | 2 (0·8%) | 18 (7%) | 1 (0·4%) |
| Hyperphosphatemia | 0 | 0 | 14 (6%) | 3 (1·2%) | 0 |
| Bacteremia | 0 | 0 | 13 (5%) | 3 (1·2%) | 1 (0·4%) |
| Decreased white | 0 | 0 | 6 (2%) | 9 (4%) | 0 |
| blood cell count | |||||
| Respiratory failure | 0 | 0 | 2 (0·8%) | 5 (2%) | 7 (3%) |
| Syncope | 0 | 0 | 13 (5%) | 0 | 0 |
| Urinary tract infection | 0 | 0 | 12 (5%) | 0 | 0 |
| Leukocytosis | 0 | 0 | 9 (4%) | 2 (0·8%) | 0 |
| Elevated blood phosphocreatine kinase | 7 (3%) | 7 (3%) | 7 (3%) | 4 (1·6%) | 0 |
| Fungal pneumonia | 0 | 0 | 11 (4%) | 0 | 0 |
| Hyperglycemia | 0 | 0 | 8 (3%) | 1 (0·4%) | 0 |
| Cellulitis | 0 | 0 | 7 (3%) | 0 | 1 (0·4%) |
| Lung infection | 0 | 0 | 7 (3%) | 0 | 1 (0·4%) |
| Mucosal inflammation | 0 | 0 | 5 (2%) | 2 (0·8%) | 0 |
| Multi-organ failure | 0 | 0 | 0 | 0 | 7 (3%) |
| QT Prolongation | 0 | 0 | 8 (3%) | 0 | 0 |
| Septic shock | 0 | 0 | 0 | 2 (0·8%) | 4 (1·6%) |
| Subdural hematoma | 0 | 0 | 4 (1·6%) | 2 (0·8%) | 0 |
| Elevated blood bilirubin | 0 | 0 | 6 (2%) | 1 (0·4%) | 0 |
| Hyperuricemia | 0 | 0 | 5 (2%) | 1 (0·4%) | 0 |
| Gastrointestinal hemorrhage | 0 | 0 | 4 (1·6%) | 1 (0·4%) | 0 |
| Increased transaminase | 0 | 0 | 6 (2%) | 0 | 0 |
| Atrial fibrillation | 0 | 0 | 5 (2%) | 0 | 0 |
| Back pain | 0 | 0 | 5 (2%) | 0 | 0 |
| Clostridium difficile colitis | 0 | 0 | 5 (2%) | 0 | 0 |
| Skin infection | 0 | 0 | 5 (2%) | 0 | 0 |
| Muscle weakness | 0 | 0 | 5 (2%) | 0 | 0 |
| Pleural effusion | 0 | 0 | 5 (2%) | 0 | 0 |
| Leukopenia | 0 | 0 | 2 (0·8%) | 3 (1·2%) | 0 |
ALT, Alanine aminotransferase; AML, acute myeloid leukemia; AST, aspartate aminotransferase; SAF, safety analysis set
Percentages ≥2% were rounded to the nearest whole number.
Adverse events listed occurred in ≥10% of patients (Grade1/2) or occurred in ≥2% of patients (Grade ≥3);
For a list of all Grade 3, 4, and 5 treatment-emergent adverse events occurring in ≤10% of patients, please refer to Table S3 in the Supplemental Appendix (pages 5–12).
Dose-associated increases in the incidences of diarrhea, pyrexia, and elevated AST were observed at gilteritinib doses ≤200mg.
Diarrhea and elevated AST were identified as dose-limiting toxicities in two of three patients in the 450mg/d dose cohort.
Reasons for treatment discontinuation are presented in Table S5 (Supplemental Appendix, page 14); progressive disease (6%; n=15/252) and sepsis (3%; n=7/252) were the most common AEs that led to study discontinuation. Drug-related TEAEs leading to discontinuation occurred in 10% of patients (n=25/252). The most common drug-related AE that led to treatment discontinuation was elevated blood creatinine phosphokinase (1.2%; n=3/252 patients). For a complete list of drug-related AEs leading to treatment discontinuation, please refer to the Supplemental Appendix (Table S6, Supplemental Appendix page 15).
A total of 95 deaths occurred in the SAF; the causes of death are listed in Table 3. Seven deaths considered possibly/probably related to treatment were due to pulmonary embolism, respiratory failure, hemoptysis, intracranial hemorrhage, ventricular fibrillation, septic shock, and neutropenia (one each); four were considered DLTs (intracranial hemorrhage, pulmonary embolism, ventricular fibrillation, and septic shock). Gilteritinib MTD was established as 300 mg when two of the three patients enrolled in the 450 mg dose-escalation cohort experienced two DLTs (diarrhea and elevated aspartate aminotransferase [AST]). In patients who received 80–300 mg/d doses of gilteritinib in the dose escalation or expansion phases, treatment-related Grade ≥3 diarrhea occurred in the 120 mg/d (n=1) and 200 mg/d (n=2) dose groups; elevated AST occurred in the 80 mg/d (n=1), 200 mg/d (n=3), and 300 mg/d (n=1) dose groups; and elevated alanine aminotransferase occurred in the 80 mg/d (n=1), 120 mg/d (n=1), and 200 mg/d (n=4) dose groups. The probability of experiencing a DLT was <20% for gilteritinib doses up to 200mg (Figure S1, Supplemental Appendix, page 19).
Table 3.
Causes of Death Following Gilteritinib Treatment
| Primary Cause of Deatha | 20 mg, n | 40 mg, n | 80 mg, n | 120 mg, n | 200 mg, n | 300 mg, n | 450 mg, n |
|---|---|---|---|---|---|---|---|
|
| |||||||
| AML | 3 | 5 | 5 | 10 | 13 | 5 | 0 |
|
| |||||||
| Anemia | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
|
| |||||||
| Bacteremia/staphylococcal bacteremia | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
|
| |||||||
| Bronchopulmonary aspergillosis | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
|
| |||||||
| Cardiac arrest | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
|
| |||||||
| Cellulitis | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
|
| |||||||
| Cerebrovascular ischemia | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
|
| |||||||
| Colitis/neutropenic colitis | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
|
| |||||||
| Death | |||||||
| Sudden death | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Death NOS | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
|
| |||||||
| Diabetic ketoacidosis | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
|
| |||||||
| Febrile neutropenia | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
|
| |||||||
| Hepatic infection | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
|
| |||||||
| Hemoptysis | 0 | 0 | 1b | 0 | 0 | 0 | 0 |
|
| |||||||
| Hypoxia | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
|
| |||||||
| Intracranial hemorrhage | 2c | 0 | 0 | 0 | 1 | 0 | 0 |
|
| |||||||
| Loss of consciousness | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
|
| |||||||
| Lung infection | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
|
| |||||||
| Multi-organ failure | 0 | 1 | 0 | 1 | 5 | 0 | 0 |
|
| |||||||
| Neutropenia | 0 | 0 | 0 | 1b | 0 | 0 | 0 |
|
| |||||||
| Pneumonia | 0 | 0 | 0 | 2 | 2 | 0 | 0 |
|
| |||||||
| Pulmonary embolism | 0 | 0 | 0 | 0 | 1b | 0 | 0 |
|
| |||||||
| Renal failure | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
|
| |||||||
| Respiratory failure | 0 | 0 | 1 | 1 | 5 | 1 | 0 |
|
| |||||||
| Sepsis/staphylococcal sepsis | 0 | 0 | 0 | 1 | 8 | 0 | 0 |
|
| |||||||
| Septic shock | 0 | 0 | 2c | 0 | 2 | 0 | 0 |
|
| |||||||
| Ventricular tachycardia | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
|
| |||||||
| Ventricular fibrillation | 0 | 0 | 0 | 1b | 0 | 0 | 0 |
AML, acute myeloid leukemia; NOS, not otherwise specified
Subjects may have multiple causes of death.
Possibly related to treatment.
One death considered possibly related to treatment.
PHARMACOKINETIC PROFILE AND PHARMACODYNAMIC EFFECTS OF GILTERITINIB
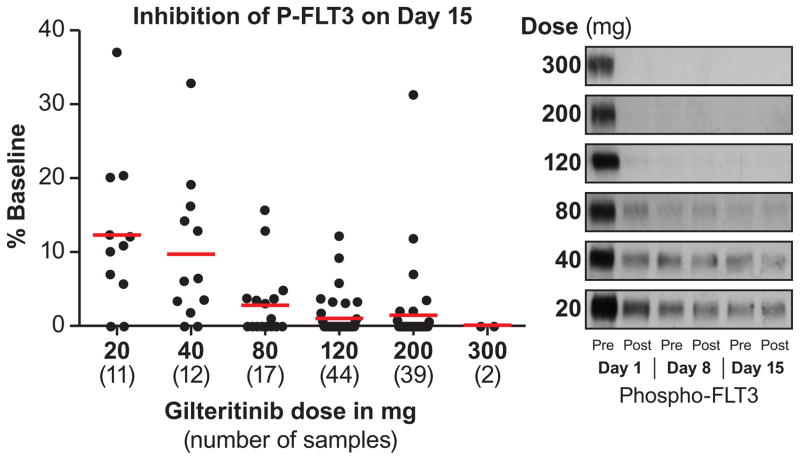
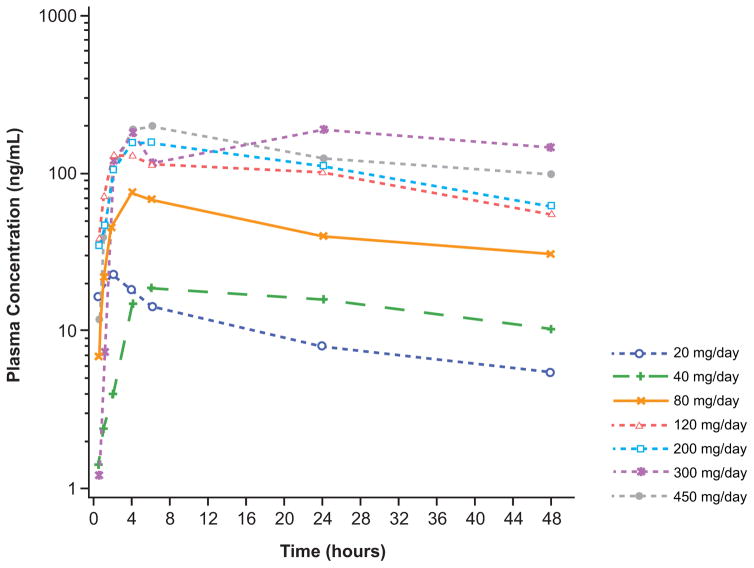
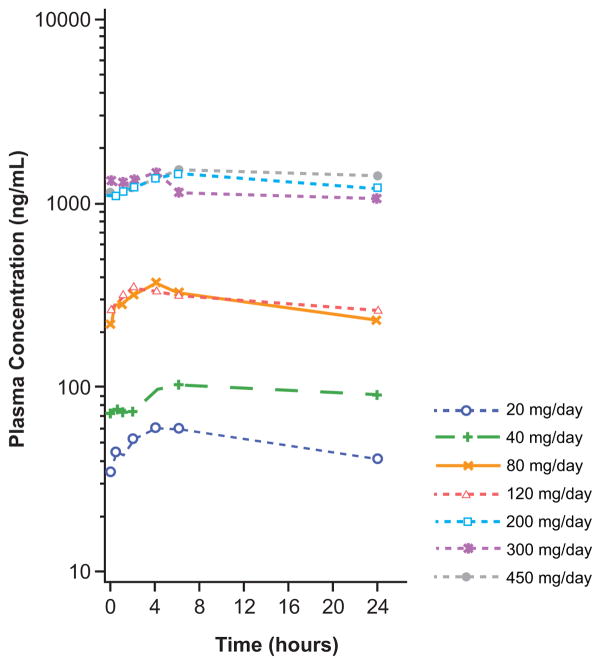
Gilteritinib plasma concentrations following oral daily dosing (20–450 mg) were generally dose proportional and showed substantial accumulation until steady state levels at treatment Day 15 (Figure 2A and 2B; Table S7, Supplemental Appendix, page 16). Potent target inhibition was observed at all dose levels, which prompted expansion of all dose cohorts ranging from 20 to 300 mg/d. An exposure-related increase in inhibition of FLT3 phosphorylation was observed with increasing gilteritinib plasma concentrations (Figure 2C): ≥90% of FLT3 phosphorylation inhibition was observed by Day 8 in the majority of patients receiving ≥80 mg. In vivo inhibition of FLT3 occurred at all dose levels (Figure 2C).
Figure 2. Gilteritinib Pharmacokinetic Profile and Pharmacodynamic Effects.
A) Gilteritinib plasma concentrations over the 24-hour dosing period following a single dose (Cycle 0 Day −2).
B) Gilteritinib plasma concentrations over the 24-hour dosing period following multiple doses (Cycle 1 Day 15).
C) Plasma inhibitory activity assay results.
Graphs depicting gilteritinib plasma concentrations over the 24-hour dosing period are representative of the following numbers of patients in each dose cohort: 20mg/d, n=4; 40mg/d, n=3; 80mg/d, n=3; 120mg/d, n=3; 200mg/d, n=2; 300mg/d, n=3; 400mg/d, n=1. Plasma samples from patients treated at different dose levels were assayed for inhibitory activity against FLT3-ITD receptors in Molm14 cells using immunoblotting as described in the Methods section. (Left) Each filled circle represents a sample from a single patient at the indicated dose level, collected immediately prior to dosing on Day 15 Cycle 1. For each dose level, the mean plasma inhibitory activity result is indicated by a red line. For each point, the reference sample was collected immediately prior to the first dose on Day 1 Cycle 1. (Right) Representative immunoblots of PIA assays from pre- and post-dose on Days 1, 8, and 15 of Cycle 1 are shown.
ANTILEUKEMIC ACTIVITY OF GILTERITINIB
As noted with the inhibition of FLT3 phosphorylation, the antileukemic effects of gilteritinib were observed in all dose cohorts. Across the entire FAS (n=249), overall response rate (ORR) was 40% (CR=8%, CRp=4%, CRi=18%, PR=10%) with a median response duration of 17 weeks (95% CI: 14, 29); three patients had relapsed with a response duration that lasted less than 1 cycle and one patient had a response duration of 55 weeks prior to relapse) and median OS of 25 weeks (95%CI: 20, 30; Table 4). The ORR in FLT3mut+ patients (49%; CR=9%, CRp=5%, CRi=22%, PR=12%; n=191) was higher than in patients with FLT3WT (12%; CR=2%, CRp=0%, CRi=7%, PR=3%; n=58; Table 4 and Table S8, Supplemental Appendix, page 17). Similarly, FLT3mut+ patients had a greater reduction from baseline in bone marrow myeloblasts than patients with FLT3WT and greater percentage reductions in bone marrow myeloblasts were seen with gilteritinib doses ≥80 mg/day (Figure S2 A and B, Supplemental Appendix, pages 20–21). Among the FLT3mut+ patients who had received any prior TKI (n=57), ORR was 37% (CR=4%; n=2/57], CRp=4%; n=2/57, CRi=18%; n=10/57], PR=11%; n=6/57). The median OS and median DOR in patients who had received any prior TKI therapy was 20 weeks (95% CI: 11, 32) and 14 weeks (95% CI: 6, 55), respectively. None of the three subjects who had received ≥2 prior TKIs responded to treatment; however, all three these patients received low doses of gilteritinib (20 mg, n=1; 40 mg, n=2). In FLT3mut+ patients who had received prior therapy with sorafenib (n=39), ORR was 49% (CR=5%; n=2/39], CRp=8%; n=3/39, CRi=26%; n=10/39, PR=10%; n= 4/39).
Table 4.
Antileukemic Response to Gilteritinib in Patients With R/R AML
| Antileukemic Response | FAS Patient Population (n=249) | FLT3WT (n=58) | FLT3mut+ | |
|---|---|---|---|---|
| All Patients (n=191) | Patients Receiving ≥80mg/d (n=169) | |||
| CR | 19 (8% [5, 12]) | 1 (2% [0, 9]) | 18 (9% [6, 15]) | 18 (11% [6, 16]) |
| CRp | 10 (4% [2, 7]) | 0 | 10 (5% [3, 9]) | 10 (6% [3, 11]) |
| CRi | 46 (19% [14, 24]) | 4 (7% [2, 17] | 42 (22% [16, 29]) | 41 (24% [18, 31]) |
| PR | 25 (10% [7, 15]) | 2 (3% [0, 12]) | 23 (12% [8, 18]) | 19 (11% [7, 17]) |
| CRca | 75 (30% [25, 36]) | 5 (9% [3, 19]) | 70 (37% [30, 44]) | 69 41% (33, 49) |
| ORRb | 100 (40% [34, 47]) | 7 (12% [5, 23]) | 93 (49% [41, 56]) | 88 (52% [44, 60]) |
| Median response durationa | 17 (14, 29) | 12 (3, 17) | 20 (14, 33) | 20 (14, 33) |
| Median overall survivalb | 25 (20, 30) | 17 (11, 21) | 30 (23, 33) | 31 (24, 59) |
CI, confidence interval; CR, complete remission; CRc, composite remission (CR+CRp+CRi); CRp, complete remission with incomplete platelet recovery; CRi, complete remission with incomplete hematologic recovery; FAS, full analysis set; PR, partial response; ORR, overall response rate (CRc+PR)
Data presented as n (% [95% CI]) unless otherwise noted.
Data presented as % (95% CI).
Data presented as weeks (95% CI).
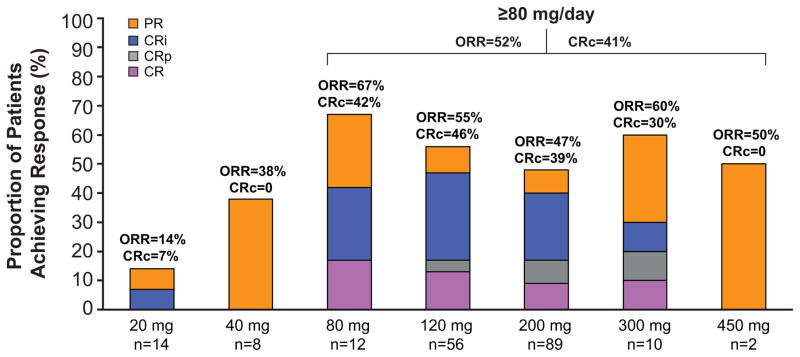
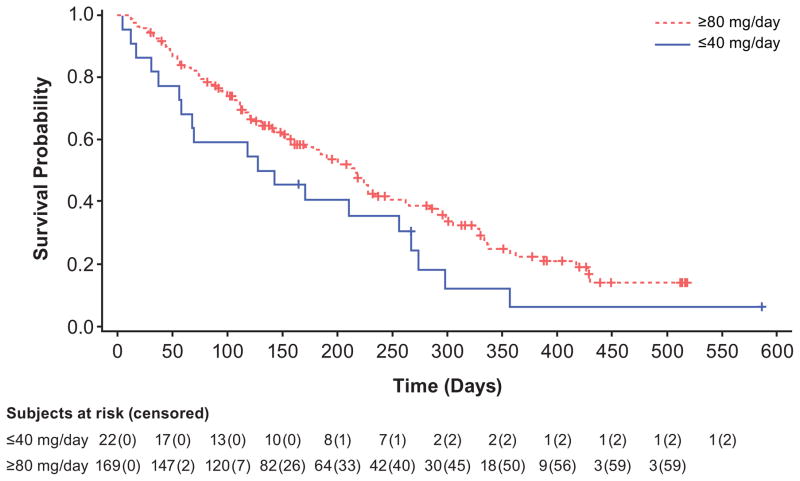
Of the 191 FLT3mut+ patients in the FAS, 70 (37%) achieved CR, CRi, or CRp; although CR, CRi, and CRp occurred at all doses (Figure 3A), responses occurred most frequently among FLT3mut+ patients receiving doses ≥80mg/d. The ORR in FLT3mut+patients receiving ≥80 mg/d was 52% (CR=11%, CRp=6%, CRi=24%, PR=11%), versus 12% for FLT3WT, with a median OS of 31 weeks (95% CI: 24, 59; Table 3 and Figure 3B) and a median response duration of 20 weeks (95% CI: 14, 33).
Figure 3. Clinical Response to Gilteritinib (≥80mg) in FLT3-Mutation-Positive Relapsed/Refractory AML Patients.
A) Overall clinical response, by dose, in patients with FLT3-mutation-positive R/R AML.
CR, complete remission; CRi, complete remission with incomplete hematologic recovery; CRp, complete remission with incomplete platelet recovery; PR, partial remission. Composite response (CRc=CR+CRi+CRp) and overall response (ORR=CRc+PR) are noted in bold type.
B) Kaplan–Meier curve showing the overall survival of patients receiving ≤40mg gilteritinib versus ≥80mg gilteritinib.
The median time to best response for all FLT3mut+ patients at dosages ≥80 mg was 7·2 weeks (95% CI: 4·3, 8·1); swimmer plots for all patients are shown in Figure S3 (Supplemental Appendix, pages 22–27). The transplant rate for the entire FLT3mut+ patient population was 19% (n=36/191); the transplant rate for all FLT3mut+ patients who received gilteritinib doses ≥80 mg/d was 19% (n=32/169). A total of 35% (n=13/37) of transplanted patients resumed gilteritinib therapy after engraftment as maintenance therapy. Landmark analyses comparing OS rates in patients who underwent HSCT (n=37) with those who did not are presented in Figure S4 (Supplemental Appendix, page 28); 36 of the 37 patients (97%) who underwent HSCT harbored FLT3 mutations. The median OS in all FLT3mut+ responders who underwent HSCT (n=32), including patients who achieved PR, was 47 weeks (95% CI: 32, 61); in FLT3mut+ responders who did not undergo HSCT (n=61), median OS was 42 weeks (95% CI: 31, 48). The overall transplant rate for FLT3mut+ patients who achieved PR or better was 34% (n=32/93). Of all FLT3mut+ patients who achieved CRc (n=70), 47 (67%) had no prior transplant; 22 of these 47 patients (47%) underwent HSCT.
Among FLT3mut+ patients, ≥80 mg gilteritinib dosages were associated with antileukemic activity in both TKI-naive and prior TKI use subgroups with more frequent responses noted in TKI-naive patients (ORR=56% vs 42%, respectively; Supplemental Appendix Table S9, page 18). At these dosages, ORRs in patients with FLT3-ITD or both FLT3-ITD and -D835 mutations were 55% and 62%, respectively; ORR in patients with FLT3-D835 mutations only was 17% (Supplemental Appendix Table S9, page 18). Of the 16 patients in the FAS who had only FLT3-D835 mutations, two responded to treatment with gilteritinib. One patient with only FLT3-D835 achieved CRi at Day 1 Cycle 2 but was not evaluable at Day 1 of Cycle 3; response duration was one day because the patient was censored at the last relapse-free disease assessment date (ie, Cycle 2 Day 1). The other patient achieved PR at Day 1 Cycle 2 and relapsed at Day 1 of Cycle 3; the DOR was 5·9 weeks.
DISCUSSION
This first-in-human, biomarker-driven study confirms that gilteritinib is a highly specific, potent FLT3 inhibitor with antileukemic activity against FLT3-ITD mutations in the presence or absence of TKD mutations. Gilteritinib was well tolerated and displayed clinical activity across a wide therapeutic window in a heavily pretreated FLT3mut+ R/R AML population. Furthermore, gilteritinib demonstrated a long elimination half-life, supporting once-daily dosing. Correlative studies confirmed consistent, sustained, and potent FLT3 kinase inhibition in patients receiving ≥80 mg/d. Responses in all FLT3mut+ patients receiving ≥80 mg/d occurred frequently and, regardless of whether patients underwent HSCT, were associated with prolonged survival (median OS, 31 weeks) compared with reported survival durations from studies of other cytotoxic salvage chemotherapy regimens (median OS, 15 to 20 weeks).26,27 The median duration of gilteritinib response in FLT3mut+ patients was 20 weeks. Because the protocol mandated early response confirmation followed by bone marrow evaluations every 8 to 12 weeks in responding patients as well as censoring of patients who died due to reasons unrelated to AML, an overestimation of response duration is unlikely.
Clinical response rates in FLT3mut+ were higher than in FLT3WT patients, which underscores the specificity of gilteritinib. Composite response rates in FLT3mut+ patients following treatment with gilteritinib were comparable with those reported for quizartinib.28 However, unlike with quizartinib, high response rates were maintained when both FLT3-ITD and D835 mutations were present.15 Median OS with gilteritinib (25 weeks) was also longer than that reported in a phase 1 study of quizartinib (study population, 14 weeks; FLT3-ITD subgroup, 18 weeks).12 The ability of gilteritinib to generate sustained responses via FLT3 inhibition, coupled with reasonable survival duration in all tested FLT3 mutation types, validates FLT3 as a therapeutic target in R/R AML. The 19% transplant rate for the FLT3mut+ population is not surprising given that several factors limit delivery of HSCT (eg, advanced age, donor availability, and prior HSCT). Almost one-third of patients in our study had undergone prior HSCT and a substantial proportion were aged >70 years. The transplant rate reported herein is similar to that reported in a comparable R/R AML population in the Cephalon 204 study.26 Notably, the transplant rate among FLT3mut+ patients who had not received prior HSCT in our study was 47%; however, receipt of HSCT after gilteritinib therapy had little impact on OS, which in our landmark analysis was similar among transplanted and nontransplanted FLT3mut+ patients (47 vs 42 weeks, respectively). While a Mantel-Byar or a Cox-Time Dependent analysis would theoretically be of value to clarify this point, due to the small number of patients who underwent transplantation in this study (n=37), these analyses are not possible. Furthermore, the small number of patients who received gilteritinib maintenance after HSCT (n=13) limits any conclusions regarding the merits of this approach outside of confirming its feasibility.
Gilteritinib toxicity was modest and manageable in outpatient settings. As expected in R/R AML, neutropenic infections were common but treatment-related mortality from neutropenia was <1%. Prolongation of QTc interval occurred in only 4% of gilteritinib-treated patients, which is lower than that reported for other FLT3 inhibitors such as quizartinib, although lower doses of quizartinib may minimize the incidence of QTc prolongation.12,28 Furthermore, the DLT rate curve and PD data support a large therapeutic window of active, tolerable gilteritinib doses ranging from 20–300 mg/d. The 120 mg/d dose was chosen as the starting dose for future studies as it can provide potent FLT3 inhibition and allow for dose modification without compromising tolerability or antileukemic effects; phase 3 testing of gilteritinib in FLT3mut+ R/R AML (NCT02421939) has been initiated.
Clinical responses were also observed in some FLT3WT patients. This may be related to AXL inhibition, other off-target TKI effects, and/or inhibition of cryptic FLT3 kinase activation (eg, from rare TKD mutations not detected by polymerase chain reaction or FLT3WT kinase activation through FLT3 ligand).29,30 Non-canonical activation of FLT3 signaling may identify additional patient populations that could benefit from gilteritinib therapy.
The pharmacodynamics-driven design of the current study led to the conclusion that gilteritinib doses ≥80 mg/d were able to potently and consistently inhibit FLT3 in vivo. The large sample size derived from combining all dose groups ≥80 mg/d allowed us to gauge the impact of gilteritinib with greater precision in this R/R AML population and to place our results in the context of standard therapy. Relapsed/refractory FLT3mut+ AML patients have infrequent and often short-lived responses to standard salvage induction chemotherapy, resulting in few long-term survivors. Response rates and OS observed with gilteritinib in this study appear better than those reported in R/R AML patients receiving standard chemotherapy.26,27 This provides a rationale for Phase 3 development as a single agent in R/R FLT3mut+ AML; this trial is ongoing (NCT02421939).
Gilteritinib is one of several FLT3-targeted inhibitors that are currently in development. Midostaurin, as a single agent, was shown to frequently reduce peripheral blood blast counts in wild-type and FLT3mut+ AML but generated limited bone marrow blast reductions and clinical response was brief before disease progression.7 Midostaurin’s lack of mutation selectivity among responders, coupled with preclinical evidence of broad kinase inhibitory activity, suggest that its efficacy may not be solely attributable to FLT3 inhibition. In a phase 2b study of twice-daily midostaurin in R/R AML, 71% of patients with FLT3 mutations and 42% of patients with FLT3WT had a reduction in blast counts; however, CR was not achieved.7 Thus, combining midostaurin with intensive cytotoxic chemotherapy or hypomethylating agents may be a better strategy than monotherapy. A survival benefit for the combination of midostaurin and intensive combination chemotherapy in newly diagnosed FLT3mut+ AML was recently reported.31,32
The benefit of combining chemotherapy with TKIs may not apply to all broad-spectrum FLT3 kinase inhibitors. Indeed, the addition of lestaurtinib to intensive chemotherapy in two British cooperative group studies of newly diagnosed FLT3mut+ AML patients under 60 years of age failed to improve CR rate, relapse-free survival, or OS, and modestly increased gastrointestinal toxicity.33 Two German cooperative group randomized, phase 2, placebo-controlled trials investigated sorafenib in combination with intensive chemotherapy for newly diagnosed AML (without selection for FLT3-ITD).34,35 The SORAML trial, conducted in patients aged <60 years, showed that the addition of sorafenib to intensive chemotherapy resulted in higher 3-year event-free survival (EFS) rates than placebo (40% and 22%, respectively; p=0.01). However, sorafenib yielded no improvement in OS and was associated with increased rates of grade ≥3 AEs.34 In the other study, sorafenib plus intensive chemotherapy in patients aged >60 years of age, failed to increase CR, EFS, or OS but was associated with treatment-related toxicity that led to therapy discontinuation.35 Neither study demonstrated statistically significant improvement in CR, EFS, or OS in FLT3-ITD subgroups, although the number of patients with FLT3-ITD mutations in both trials was small.34,35 Evidence suggests that administration of sorafenib as a single agent after allogeneic HSCT may enhance treatment response in patients with FLT3-ITD mutations.36
While there is continued interest in combining FLT3 inhibitors with chemotherapy, there is also an interest in more potent FLT3 inhibitors with greater selectivity and reduced toxicity. Two such agents are quizartinib and crenolanib. Although quizartinib, a potent and selective once-daily FLT3 inhibitor, resulted in high CRc and ORR in R/R AML, durable survival generally necessitated HSCT37 and non-transplanted patients frequently developed resistance due to treatment-emergent FLT3-TKD mutations (eg, D835).38 In the phase 1 study of quizartinib, median OS was 14 weeks for the entire study population and 18 weeks for patients with FLT3-ITD mutations.12 Two small, single-institution trials of crenolanib demonstrated significant antileukemic activity in R/R FLT3mut+ AML.39,40 However, due to crenolanib’s short half-life, thrice-daily dosing was required; CR or CRp rarely occurred, and nausea, vomiting, and hepatic toxicity were common.39,40
The main limitations of this study are that it was a non-randomized, single-arm, open-label study with a relatively small number of patients, which limits generalization of the findings to the AML population at large. Gilteritinib effectively inhibited FLT3 in most patients at all tested doses. As such, the small number of patients with submaximal target inhibition makes it challenging to prove that the potency of FLT3 inhibition predicts the depth of marrow response.
More FLT3mut+ patients achieved CRi than CR or CRp, suggesting that minimal residual disease persisted following gilteritinib therapy. It should be noted that all potent FLT3 inhibitors have a propensity toward generating CRis in FLT3mut+ R/R AML patients; the reasons for this outcome warrant further investigation. Some FLT3 inhibitors (eg, quizartinib) are also potent c-Kit inhibitors with substantial myelosuppression effects.12,28 In contrast, FLT3 inhibition induces terminal granulocytic differentiation of leukemic blasts in most responders, which may explain robust neutrophil recovery.41 Generation of erythrocytes and platelets in responders to FLT3 inhibitors presumably occurs from background hematopoiesis rather than differentiation. Erythropoiesis and megakaryopoiesis are frequently and persistently suppressed in the context of massive clonal expansion of FLT3mut+ cells undergoing differentiation during FLT3 inhibitor response. Finally, it is likely that differentiation abnormalities in pre-leukemic clones limit production of all hematopoietic lineages, especially among patients who were previously diagnosed with myelodysplastic syndrome. Therefore, administration of a drug that selectively targets a single mutation arising from a genetically complex and clonally diverse bone marrow environment, will likely unmask the underlying disordered hematopoietic capacity of the remaining bone marrow cells.
Changes in allelic burden during therapy were not formally investigated here, although evidence suggests that many patients respond to FLT3 inhibitors via clonal differentiation rather than a direct cytotoxic response that clears the FLT3-mutated cells from the marrow.41 Although FLT3-ITD and D835 are both activating and transforming oncogenes, in vitro data suggest that they activate different downstream signaling networks.42 These signaling differences could contribute to varying level of oncogene addiction in the R/R setting and thus different clinical effects in the setting of kinase inhibition, specifically lower response rates among patients with FLT3-D835 only. However, while it is possible that FLT3-D835 in the absence of FLT3-ITD is a weaker driver and generates a lesser degree of oncogene addiction, the small number of patients with D835 mutations in this study limits the strength of any conclusions regarding the antileukemic activity of gilteritinib in this population due to the large CI. Finally, the potential impact of additional mutations on the depth of response or development of primary acquired resistance to gilteritinib requires further elucidation.
In conclusion, gilteritinib monotherapy was well tolerated, generated high response rates and durable survival in FLT3mut+ R/R AML patients, including those with both FLT3-ITD and D835 mutations. Because gilteritinib as a single agent is likely to have limited curative capacity even when used early in the disease course, studies that integrate gilteritinib into front-line chemotherapy regimens are underway. These include trials of gilteritinib in combination with standard induction chemotherapy and high-dose cytarabine consolidation therapy (NCT02236013) or with azacitidine (NCT02752035). A placebo-controlled trial of gilteritinib as maintenance therapy for patients in first remission receiving allogeneic HSCT (NCT02997202) will be initiated. Overall, our study both confirms that FLT3 is a high-value target in R/R AML and demonstrates that long-term success of therapeutic FLT3 inhibition is optimized by agents with potent, selective, and sustained activity against FLT3-ITD mutations as well as TKD mutations.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
Despite the major advances in our understanding of leukemogenesis, effective treatment of acute myeloid leukemia (AML) remains a challenge due to disease heterogeneity, drug toxicity, and treatment resistance. For patients with an adverse genetic risk profile, relapse occurs frequently and prognosis thereafter remains bleak. Activating mutations in fms-like tyrosine kinase 3 (FLT3) have been correlated with an increased risk of relapse and subsequent poor survival due to chemotherapy resistance. Therefore, inhibition of FLT3 activity is a key therapeutic strategy in AML. A search of Medline and PubMed databases with no established date limit was conducted using the search terms ‘FLT3 inhibitors’, ‘sorafenib’, and ‘midostaurin’ in the context of clinical studies in AML, yielded 40 publications; meeting abstracts, review articles, meta-analyses, and case studies were excluded. The majority of clinical studies of FLT3 tyrosine kinase inhibitors were small-scale, single-arm, nonrandomized Phase 1 or Phase 2 studies. Of the limited number of published placebo-controlled Phase 3 studies, none had examined the effects of single-agent FLT3 tyrosine kinase inhibitors. Phase 3 studies of lestaurtinib examined the agent in combination frontline or salvage therapy regimens, and showed no statistically significant clinical response or survival benefit. It is also important to note that enrichment for FLT3 mutations is lacking in many clinical studies of FLT3 inhibitors. Furthermore, the current clinical literature is limited to first-generation FLT3 inhibitors that have limited specificity, short-lived antileukemic activity, and the potential for toxicity stemming from off-target effects. Several second-generation FLT3 inhibitors have been developed, which partially addresses these limitations. However, published data show persistent toxicity concerns as well as frequent generation of treatment emergent FLT3 tyrosine kinase domain mutations that confer resistance and limit the durability of observed clinical benefits. Particularly, FLT3-D835 mutations stand out as a frequent and vexing mechanism of resistance to FLT3 inhibitors.
Added value of this study
Gilteritinib is a highly potent and selective tyrosine kinase inhibitor with in vitro activity against FLT3 internal tandem duplication mutations and tyrosine kinase domain mutations. The current phase 1/2 study of once-daily oral gilteritinib had an innovative pharmacodynamic-driven trial design that enabled rapid identification of optimal dosing in a FLT3 mutation-enriched R/R AML population. Our results demonstrated that gilteritinib was generally well tolerated and produced sustained potent FLT3 inhibition, which correlated with frequent clinical responses and promising survival data. Response extended to patients predicted to be resistant to this class of agents, such as those with both FLT3-ITD and D835 mutations, as well as those with prior FLT3 inhibitor exposure. Findings from this study lay the foundation for phase 3 studies comparing gilteritinib against standard chemotherapy or other targeted agents.
Implications of all the available evidence
Emerging targeted therapies offer promise in patients with R/R AML. Disease in these patients is unlikely to durably respond to conventional chemotherapy regimens. As demonstrated in the current study, a potent, highly selective FLT3 inhibitor with activity against common resistance mechanisms allows for better tolerability and a sustained treatment response. These data convincingly validate FLT3 as a high-value therapeutic target in AML.
Acknowledgments
We would like to acknowledge all investigators, coordinators, and study site personnel, as well as patients and their families for their participation in this study. This research was sponsored by Astellas Pharma, Inc. (Northbrook, IL) and by a National Cancer Institute Leukemia Specialized Program of Research Excellence grant (CA100632) awarded to Drs Mark Levis and Jorge Cortes. Dr. Andreas Neubauer would also like to acknowledge his grant support from the Jose Carreras Foundation (AH06-01). Financial support for the development of this manuscript, including writing and editorial assistance under the authors’ guidance, was provided by Drs Kalpana Vijayan and Regina Switzer of SuccinctChoice Medical Communications (Chicago, IL) and was funded by the study sponsor.
CONTRIBUTIONS
AP, JA, JC, EB, CL, and S Gill contributed to the study design and were responsible for acquisition, analysis, and interpretation of the data as well as drafting and critical review of the manuscript. M Levis, M Litzow, MB, S Goldberg, CS, GS, EW, and RT contributed to the study design, data acquisition, and to the development and critical review of the manuscript. HE, ER, and CU were responsible for acquisition of data and contributed to the development and critical review of the manuscript. JJ, AN, and RL contributed to data acquisition and interpretation, and were involved in the development and critical review of the manuscript. EW, SS DC, GM, and AS were involved in patient accrual, data collection and interpretation, and the development and critical review of the manuscript. CR contributed to the interpretation of the data and the development and critical review of the manuscript.
DECLARATION OF INTERESTS
AP reports personal fees and non-financial support from Astellas US Pharma Inc, personal fees from Daichi Sankyo, personal fees and non-financial support from Arog Pharmaceuticals, personal fees from Asana Biosciences, personal fees from Actinium Pharmaceuticals, outside the submitted work. JA reports other from Astellas, non-financial support from Astellas, during the conduct of the study; other from BMS, other from Novartis, other from Spectrum, other from Ariad, other from Seattle Genetics, outside the submitted work. CL and EB are both employees of Astellas Pharma, Inc.; S Gill was a former employee of Astellas Pharma, Inc. during the conduct of the study. JC reports grants from Astellas, during the conduct of the study; grants and personal fees from Daiichi, grants from Arog, grants and personal fees from Novartis, grants and personal fees from Ariad, outside the submitted work. HE reports grants from Astellas, during the conduct of the study; personal fees from Novartis Pharmaceuticals, personal fees from Incyte Corporation, personal fees from Celgene Corporation, personal fees from Sunesis, grants from Millenium, grants from Seattle Genetics, grants from Amgen, grants from Celator, grants from Astellas, personal fees from Seattle Genetics, outside the submitted work. S Goldberg reports grants from Astellas, during the conduct of the study; grants from Ambit, grants from Celator, grants from Cyclacel, grants from Pfizer, outside the submitted work. JJ reports grants from Astellas Pharma, Inc., during the conduct of the study; grants from Daiichi-Sankyo, from Actinium Pharmaceuticals, Inc., grants and personal fees from Seattle Genetics, grants from Kura Oncology, grants and non-financial support from Celgene Corporation, grants and non-financial support from Ambit Biosciences, grants and non-financial support from TetraLogic Pharmaceuticals, personal fees from Novartis, personal fees from Bayer, personal fees from Alexion Pharmaceuticals, personal fees from Merck & Co, personal fees from Amgen, personal fees from Sunesis Pharmaceuticals, grants from Syros Pharmaceuticals, personal fees from Incyte Corporation, outside the submitted work. SS reports other from Alexion, other from Baxalta, other from Boehringer-Ingelheim, personal fees from Astellas, other from CTI BioPharma, grants, personal fees and other from Sunesis, other from Tolero, personal fees from Daiichi-Sankyo, outside the submitted work. CR reports personal fees from Amgen, personal fees from BMS, personal fees from Celgene, personal fees from Janssen, personal fees from Novartis, personal fees from Roche, personal fees from Takeda, non-financial support from Gilead, grants from Bayer, outside the submitted work. ER reports other support from Novartis, other from Incyte, other from Celgene Corporation, other from Onyx, other from Ariad, outside the submitted work. CS reports grants from Astellas, during the conduct of the study; grants from Plexxikon, Inc., outside the submitted work. GM reports personal fees from Pfizer, personal fees from Ariad, personal fees from Merck, personal fees from Sharp & Dohme, personal fees from Roche, outside the submitted work. RL reports grants and other support from Astellas Pharma, Inc., during the conduct of the study. GS reports grants from Astellas Pharma, during the conduct of the study; other from Astellas Pharma, outside the submitted work. M Levis reports grants from National Cancer Institute, USA, other from Astellas Pharma, Inc., during the conduct of the study; other from Daiichi-Sankyo, other from Novartis, grants and other from Arog Pharmaceuticals, grants and other from Ambit Biosciences, grants and other from Takeda Pharamaceuticals, outside the submitted work. AS reports grants from Astellas, during the conduct of the study; grants from Roche, grants from Novartis, grants from Clovis, grants from Gritstone Oncology, grants from Ariad, grants from BMS, grants from Medimmune, grants from ADCT, outside the submitted work. RT and M Litzow report grants from Astellas Pharma, during the conduct of the study. DC reports grants from, outside the submitted work. AN, MB, and CU have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cancer Genome Atlas Research N. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059–74. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–35. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 3.Levis M. FLT3 mutations in acute myeloid leukemia: what is the best approach in 2013? Hematology Am Soc Hematol Educ Program. 2013;2013:220–6. doi: 10.1182/asheducation-2013.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakao M, Yokota S, Iwai T, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10(12):1911–8. [PubMed] [Google Scholar]
- 5.Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97(8):2434–9. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 6.Smith BD, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103(10):3669–76. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 7.Fischer T, Stone RM, Deangelo DJ, et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28(28):4339–45. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiedler W, Serve H, Dohner H, et al. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105(3):986–93. doi: 10.1182/blood-2004-05-1846. [DOI] [PubMed] [Google Scholar]
- 9.O’Farrell AM, Yuen HA, Smolich B, et al. Effects of SU5416, a small molecule tyrosine kinase receptor inhibitor, on FLT3 expression and phosphorylation in patients with refractory acute myeloid leukemia. Leuk Res. 2004;28(7):679–89. doi: 10.1016/j.leukres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Borthakur G, Kantarjian H, Ravandi F, et al. Phase I study of sorafenib in patients with refractory or relapsed acute leukemias. Haematologica. 2011;96(1):62–8. doi: 10.3324/haematol.2010.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang W, Konopleva M, Shi YX, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100(3):184–98. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 12.Cortes JE, Kantarjian H, Foran JM, et al. Phase I study of quizartinib administered daily to patients with relapsed or refractory acute myeloid leukemia irrespective of FMS-like tyrosine kinase 3-internal tandem duplication status. J Clin Oncol. 2013;31(29):3681–7. doi: 10.1200/JCO.2013.48.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metzelder S, Wang Y, Wollmer E, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood. 2009;113(26):6567–71. doi: 10.1182/blood-2009-03-208298. [DOI] [PubMed] [Google Scholar]
- 14.Ravandi F, Alattar ML, Grunwald MR, et al. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood. 2013;121(23):4655–62. doi: 10.1182/blood-2013-01-480228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith CC, Wang Q, Chin CS, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485(7397):260–3. doi: 10.1038/nature11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Man CH, Fung TK, Ho C, et al. Sorafenib treatment of FLT3-ITD(+) acute myeloid leukemia: favorable initial outcome and mechanisms of subsequent nonresponsiveness associated with the emergence of a D835 mutation. Blood. 2012;119(22):5133–43. doi: 10.1182/blood-2011-06-363960. [DOI] [PubMed] [Google Scholar]
- 17.Alvarado Y, Kantarjian HM, Luthra R, et al. Treatment with FLT3 inhibitor in patients with FLT3-mutated acute myeloid leukemia is associated with development of secondary FLT3-tyrosine kinase domain mutations. Cancer. 2014;120(14):2142–9. doi: 10.1002/cncr.28705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee LY, Hernandez D, Rajkhowa T, et al. Pre-clinical studies of gilteritinib, a next-generation FLT3 inhibitor. Blood. 2017;129(2):257–60. doi: 10.1182/blood-2016-10-745133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neubauer A, Fiebeler A, Graham DK, et al. Expression of axl, a transforming receptor tyrosine kinase, in normal and malignant hematopoiesis. Blood. 1994;84(6):1931–41. [PubMed] [Google Scholar]
- 20.Park IK, Mishra A, Chandler J, Whitman SP, Marcucci G, Caligiuri MA. Inhibition of the receptor tyrosine kinase Axl impedes activation of the FLT3 internal tandem duplication in human acute myeloid leukemia: implications for Axl as a potential therapeutic target. Blood. 2013;121(11):2064–73. doi: 10.1182/blood-2012-07-444018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park IK, Mundy-Bosse B, Whitman SP, et al. Receptor tyrosine kinase Axl is required for resistance of leukemic cells to FLT3-targeted therapy in acute myeloid leukemia. Leukemia. 2015;29(12):2382–9. doi: 10.1038/leu.2015.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori M, Kaneko N, Ueno Y, et al. ASP2215, a novel FLT3/AXL inhibitor: preclinical evaluation in acute myeloid leukemia (AML) J Clin Oncol. 2014;32(5s suppl) abstract 7070. [Google Scholar]
- 23.Levis M, Brown P, Smith BD, et al. Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood. 2006;108(10):3477–83. doi: 10.1182/blood-2006-04-015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 25.Yin G, Li Y, Ji Y. Bayesian dose-finding in phase I/II clinical trials using toxicity and efficacy odds ratios. Biometrics. 2006;62(3):777–84. doi: 10.1111/j.1541-0420.2006.00534.x. [DOI] [PubMed] [Google Scholar]
- 26.Levis M, Ravandi F, Wang ES, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood. 2011;117(12):3294–301. doi: 10.1182/blood-2010-08-301796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roboz GJ, Rosenblat T, Arellano M, et al. International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol. 2014;32(18):1919–26. doi: 10.1200/JCO.2013.52.8562. [DOI] [PubMed] [Google Scholar]
- 28.Cortes JE, Tallman MS, Schiller G, et al. Results of a phase 2 randomized, open-label, study of lower doses of quizartinib (AC220; ASP2689) in subjects with FLT3-ITD positive relapsed or refractory acute myeloid leukemia (AML) Blood. 2013;122(494) [Google Scholar]
- 29.Reindl C, Bagrintseva K, Vempati S, et al. Point mutations in the juxtamembrane domain of FLT3 define a new class of activating mutations in AML. Blood. 2006;107(9):3700–7. doi: 10.1182/blood-2005-06-2596. [DOI] [PubMed] [Google Scholar]
- 30.Zheng R, Levis M, Piloto O, et al. FLT3 ligand causes autocrine signaling in acute myeloid leukemia cells. Blood. 2004;103(1):267–74. doi: 10.1182/blood-2003-06-1969. [DOI] [PubMed] [Google Scholar]
- 31.Gallogly MM, Lazarus HM. Midostaurin: an emerging treatment for acute myeloid leukemia patients. J Blood Med. 2016;7:73–83. doi: 10.2147/JBM.S100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strati P, Kantarjian H, Ravandi F, et al. Phase I/II trial of the combination of midostaurin (PKC412) and 5-azacytidine for patients with acute myeloid leukemia and myelodysplastic syndrome. Am J Hematol. 2015;90(4):276–81. doi: 10.1002/ajh.23924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knapper S, Russell N, Gilkes A, et al. A randomized assessment of adding the kinase inhibitor lestaurtinib to first-line chemotherapy for FLT3-mutated AML. Blood. 2017;129(9):1143–54. doi: 10.1182/blood-2016-07-730648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Röllig C, Serve H, Huttmann A, et al. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2015;16(16):1691–9. doi: 10.1016/S1470-2045(15)00362-9. [DOI] [PubMed] [Google Scholar]
- 35.Serve H, Krug U, Wagner R, et al. Sorafenib in combination with intensive chemotherapy in elderly patients with acute myeloid leukemia: results from a randomized, placebo-controlled trial. J Clin Oncol. 2013;31(25):3110–8. doi: 10.1200/JCO.2012.46.4990. [DOI] [PubMed] [Google Scholar]
- 36.Metzelder SK, Schroeder T, Finck A, et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia. 2012;26(11):2353–9. doi: 10.1038/leu.2012.105. [DOI] [PubMed] [Google Scholar]
- 37.Levis MJ, Martinelli G, Perl AE, et al. The benefit of treatment with quizartinib and subsequent bridging to HSCT for FLT3-ITD(+) patients with AML. J Clin Oncol. 2014;32(5s) [Google Scholar]
- 38.Wander SA, Levis MJ, Fathi AT. The evolving role of FLT3 inhibitors in acute myeloid leukemia: quizartinib and beyond. Ther Adv Hematol. 2014;5(3):65–77. doi: 10.1177/2040620714532123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Randhawa J, Kantarjian HM, Borthakur G, et al. Results of a Phase II Study of Crenolanib in Relapsed/Refractory Acute Myeloid Leukemia Patients (Pts) with Activating FLT3 Mutations. Blood. 2014;124(21) abstract 389. [Google Scholar]
- 40.Cortes JE, Kantarjian HM, Kadia TM, et al. Crenolanib besylate, a type I pan-FLT3 inhibitor, to demonstrate clinical activity in multiply relapsed FLT3-ITD and D835 AML. J Clin Oncol. 2016;34(Suppl) Abstract 7008. [Google Scholar]
- 41.Sexauer A, Perl A, Yang X, et al. Terminal myeloid differentiation in vivo is induced by FLT3 inhibition in FLT3/ITD AML. Blood. 2012;120(20):4205–14. doi: 10.1182/blood-2012-01-402545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leischner H, Albers C, Grundler R, et al. SRC is a signaling mediator in FLT3-ITD- but not in FLT3-TKD-positive AML. Blood. 2012;119(17):4026–33. doi: 10.1182/blood-2011-07-365726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.