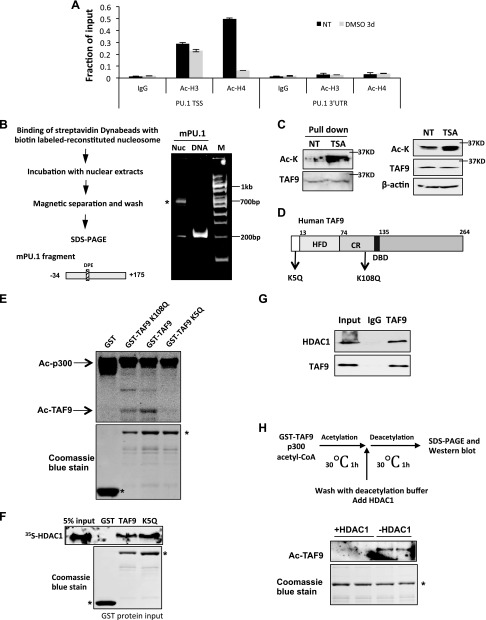
Figure 2.
TAF9 is acetylated on PU.1 core promoter, interacts with HDAC1, and is deacetylated by HDAC1. A) The recruitment of acetyl-histone H3 and -4 on mouse PU.1 promoter. ChIP assay with acetyl-H3 (Ac-H3) and acetyl-H4 (Ac-H4) Abs was performed in MEL cells induced with DMSO for 0 and 3 d. The resulting precipitated DNA was analyzed by using real-time PCR. Data are represented as means ± sem. B) Schematic representation of biotin-labeled nucleosome pull-down assay (left). Reconstituted nucleosome and control DNA were resolved in 5% native gel (right). Asterisk indicates reconstituted nucleosome. C) Proteins that bound to in vitro reconstituted PU.1 nucleosome from MEL nuclear extract treated with or without TSA were Western blotted with anti–acetyl-lysine (Ac-K) and anti-TAF9 Abs. The acetylated band colocalized with the TAF9 band. D) Schematic representation of TAF9 protein. K5 and K108 are reported acetylation sites. E) GST, GST-TAF9, K5Q, and K108Q mutants were purified from Escheria coli and incubated with p300 and 3H-labeled acetyl-CoA. The reaction mix was then subjected to SDS-PAGE and autoradiography. F) GST pull-down assay was performed by incubating immobilized GST, GST-TAF9, or K5Q mutant with in vitro translated [35S]-labeled HDAC1. The bond protein fraction was eluted and subjected to SDS-PAGE and autoradiography. G) K562 cell nuclear extract was immunoprecipitated with TAF9 Ab. The precipitated proteins were Western blotted with indicated Abs. H) Illustration of procedures of acetylation coupled deacetylation assay (top). In vitro acetylated TAF9 was incubated with or without HDAC1 (bottom). The reaction mix was Western blotted with acetyl-lysine Ab. CR, conserved region; DBD, DNA binding domain; HFD, histone fold domain; NT, no treatment; TSS, transcription start site; UTR, untranslated region.