Fig. 1.
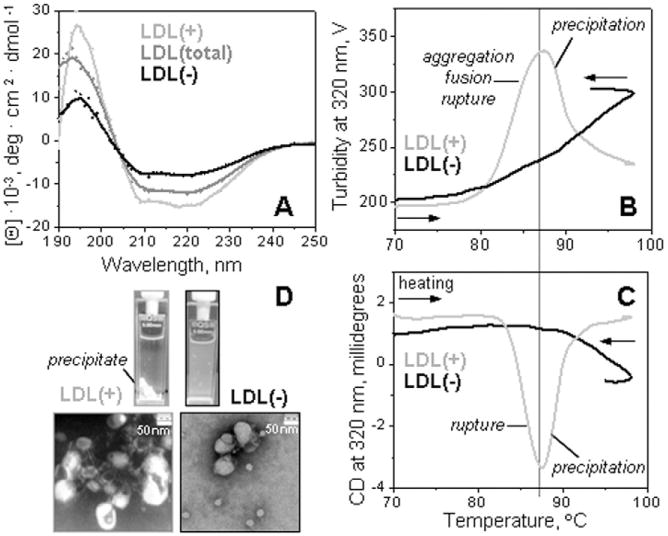
Secondary structure and thermal stability of LDL fractions monitored by circular dichroism spectroscopy. Far-UV CD spectra of intact LDL(total), LDL(+), and LDL(−) (A). The melting data recorded of LDL(+) and LDL(−) at 320 nm by turbidity to monitor changes in the particle size (B) and by near-UV CD to monitor lipid re-packing upon lipoprotein disintegration and release of core lipids (C). The data were recorded using LDL samples under standard conditions (0.35 mg protein/mL, 20 mM Na phosphate buffer, pH 7.0). The melting data in panels B and C were recorded simultaneously during sample heating and cooling at a constant rate of 11 °C/h. Steep decline in CD and turbidity amplitude observed upon heating of LDL(+) above 88 °C is due to sample precipitation (D). The melting data of LDL(total) (not shown) were similar to those of LDL(+) within the error of their experimental determination.
