Abstract
The facet joint is a common source of neck pain, particularly after excessive stretch of its capsular ligament. Peptidergic afferents have been shown to have an important role in the development and maintenance of mechanical hyperalgesia, dysregulated nociceptive signaling, and spinal hyperexcitability that develop after mechanical injury to the facet joint. However, the role of non-peptidergic isolectin-B4 (IB4) cells in mediating joint pain is unknown. Isolectin-B4 saporin (IB4-SAP) was injected into the facet joint to ablate non-peptidergic cells, and the facet joint later underwent a ligament stretch known to induce pain. Behavioral sensitivity, thalamic glutamate transporter expression, and thalamic hyperexcitability were evaluated up to and at day 7. Administering IB4-SAP prior to a painful injury prevented the development of mechanical hyperalgesia that is typically present. Intra-articular IB4-SAP also prevented the upregulation of the glutamate transporters GLT-1 and EAAC1 in the ventral posterolateral nucleus of the thalamus and reduced thalamic neuronal hyperexcitability at day 7. These findings suggest that a painful facet injury induces changes extending to supraspinal structures and that IB4-positive afferents in the facet joint may be critical for the development and maintenance of sensitization in the thalamus after a painful facet joint injury.
Keywords: pain, facet joint, neuronal hyperexcitability, glutamate transporter, brain
1. Introduction
The facet joint is a common source of neck pain [1], and excessive stretch of that joint’s capsular ligament beyond its physiologic limit can produce painful injury [2–5]. The facet capsule is innervated by both peptidergic and non-peptidergic afferents [6–11], which are activated when it undergoes stretch [3,12], initiating nociceptive signaling and spinal neuronal hyperexcitability [5,13–15]. The involvement of peptidergic neurons in facet joint-mediated pain has been previously been investigated [13,15]. Approximately one third of peptidergic neurons are also positive for isolectin-B4 (IB4) [16–18]. IB4-positive cells have been shown to contribute to inflammatory and neuropathic pain, and their ablation prevents the development of behavioral sensitivity in models of musculoskeletal pain, spinal nerve ligation, and cancer pain [19–21]. Despite the focus on peptidergic afferents in pain, the relationship between non-peptidergic cells that bind IB4 in the facet joint and its painful injury is unknown.
Dysregulation of the glutamate system is one of the hallmarks of central sensitization in the central nervous system. Glutamate receptors and transporters are highly regulated throughout the spinal cord and thalamus in arthritis [26] and joint pain [23,27,28]. Specifically, the glutamate transporter (GLT-1) and excitatory amino acid carrier (EAAC1) are both downregulated in the spinal cord after painful facet joint injury [23,27,28]. The glutamate system contributes to spinal neuronal hyperexcitability [28,29]. Nociceptive information in the spinal cord is relayed to the thalamus before transmission to other brain structures [30], and altered thalamic neuronal firing has been detected in studies of inflammatory and neuropathic pain [31–33]. Further, many aspects of central sensitization and neuronal hyperexcitability are evident in the spinal cord early and later after painful facet joint injury [5,15,28,34,35]. However, despite documentation of a host of robust peripheral [14,36–39] and spinal changes that may contribute to pain, the supraspinal changes have not yet been evaluated for pain from the facet or traumatic injury to other joints.
Since IB4-positive neurons may play a role in pain, removing the non-peptidergic afferents that innervate the cervical facet joint is hypothesized to modify aspects of the brain’s responses that are involved in the central sensitization that occurs with facet pain. As such, we first evaluated if behavioral sensitivity after a mechanical facet joint injury is modified after targeted chemical ablation of IB4-positive cells with a saporin agent. Based on those findings, a follow up study assessed neuronal hyperexcitability in the thalamus using electrophysiological recordings to examine if ablating IB4-positive cells in the facet joint prior to its injury alters evoked neuronal responses in the thalamus at day 7 after injury. Similarly, GLT-1 and EAAC1 were also quantified by immunohistochemistry in the ventral posterolateral nucleus (VPL) of the thalamus at that same time after injury in groups with and without non-peptidergic joint afferents present.
2. Material and Methods
2.1 Experimental design
Collectively studies evaluated the effects of ablating non-peptidergic neurons on behavioral outcomes, thalamic neuronal hyperexcitability, and glutamatergic responses in the VPL in an established rat model of painful facet joint distraction [13,14,40,41]. All studies used male Holtzman rats (Harlan Sprague-Dawley; Indianapolis, IN) weighing 380–430g and housed under USDA- and AAALAC-compliant conditions, with a 12–12 hour light-dark cycle and free access to food and water. Experimental procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee and carried out under the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain [42].
In the first study, behavioral sensitivity was characterized following the ablation of non-peptidergic neurons in the C6/C7 bilateral cervical facet joints prior to imposing a painful facet joint distraction at that same joint level. Rats received a bilateral intra-articular injection in the C6/C7 facet joints of either IB4-saporin (IB4-SAP; n=16) or vehicle saporin (n=11) using previously reported methods [15,28,43]. Fourteen days later, rats underwent either a painful facet joint distraction or sham surgical procedure (n=11 vehicle injury; n=12 IB4-SAP injury; n=4 IB4-SAP sham). Forepaw mechanical hyperalgesia was measured at baseline (day 0) before surgery and on days 1, 3, 5, and 7 after surgery.
Neuronal hyperexcitability was measured in the VPL of the thalamus on day 7 after painful facet joint injury (n=4) or sham (n=3) surgery, without any joint injection, to define thalamic neural firing in response to the injury or sham surgery alone. Building on the behavioral findings from the first study, thalamic neuronal firing also was measured on day 7 in separate groups of rats receiving IB4-SAP and either injury (n=6; IB4-SAP injury) or sham (n=5; IB4-SAP sham) procedures. Brain tissue was harvested from subsets of the rats from the behavioral and electrophysiology studies at day 7 (n=9 vehicle injury; n=12 IB4-SAP injury; n=4 IB4-SAP sham, n=3 sham) to evaluate changes in glutamate transporter expression in the thalamus using immunohistochemistry.
2.2 Intra-articular injections
Neurons that bind IB4 are typically non-peptidergic [44,45] and are involved in pain from nerve injury and inflammation [19,46]. In order to identify the contribution, if any, of that population of sensory neurons in the joint for facet stretch-induced pain, rats received intra-articular injections of 5μg of saporin conjugated to isolectin-B4 (IB4-SAP; Advanced Targeting Systems; San Diego, CA; n=27) in 10μL of PBS to ablate the IB4-binding neurons. That 5μg dose of IB4-SAP was selected from a previously published report as sufficient to ablate non-peptidergic afferents [19] and is the same as the dose used in a previous study of facet joint-mediated pain [15]. Separate rats received unconjugated saporin (vehicle; Advanced Targeting Systems; San Diego, CA, n=18) to serve as controls. Either IB4-SAP or vehicle saporin were administered using established methods for intra-articular injection [15,28,43].
All surgical procedures were performed under inhalation isoflurane anesthesia (4% for induction, 2.5% for maintenance). Briefly, the bilateral C6/C7 facet joints were exposed. A 33-gauge beveled needle attached to a 10μL syringe (Hamilton Company; Reno, NV) was advanced manually into the facet joint using a dorsal approach, and 5μg of IB4-SAP or vehicle saporin in 10μL of PBS was slowly injected into the facet joint. Following joint injections, wounds were closed with polyester suture and surgical staples, and rats were recovered in room air.
2.3 Surgical procedures for facet joint distraction
Because saporin induces cell death within 14 days [13,15,47], the facet joint injury or sham procedure was performed 14 days after the joint injection. Under inhalation isoflurane anesthesia, rats underwent either a facet joint distraction or sham procedure as previously described [13,14,28,40,41]. Briefly, the C6 and C7 laminae were exposed and attached to a loading device via microforceps; the C6 vertebra was displaced 0.7mm rostrally while the C7 vertebra was held in place. A camera mounted to a surgical scope tracked markers on the C6 and C7 laminae and the capsular ligament during injury to measure the severity of the injury. The relative displacement of the C6 and C7 vertebrae, corresponding capsular ligament distraction and tensile strain across the capsule during distraction were quantified. Displacement and strain values are reported as the mean±standard deviation and were compared between the IB4-SAP and vehicle injury groups using a Student’s t-test to compare the injury severity between injury groups.
An additional group of rats underwent the same sham surgical procedures with device attachment only but no joint distraction. Injury or sham only rats in the electrophysiology study underwent the same surgical procedures described but did not have prior joint injection. Following all surgeries, incisions were closed and rats were recovered in room air.
2.4 Behavioral assessment of mechanical hyperalgesia
Bilateral forepaw mechanical hyperalgesia was evaluated in all rats using a modified version of Chaplan’s thresholding method [5,22,48,49]. Forepaw withdrawal thresholds were measured for each rat at baseline (day 0) before surgery and on days 1, 3, 5 and 7 following facet joint distraction or sham surgery using a series of nine von Frey filaments with increasing strengths (0.4g, 0.6g, 1.4g, 2g, 4g, 6g, 8g, 15g, 26.0g) (Stoelting Co.; Wood Dale, IL). To measure mechanical hyperalgesia, a filament was recorded as the response threshold if the next higher filament also induced a positive response [14,48]. Each testing session consisted of 5 stimulations to each paw over 3 rounds of stimulation, and the average threshold was recorded for each paw. Responses between left and right forepaws were compared using a paired t-test and combined to obtain a single average for each rat on each day. A repeated measures ANOVA was used to detect any differences between groups in the overall hyperalgesia; differences on each day were detected using a one-way ANOVA with Bonferroni correction between groups. All threshold data are reported as the mean±standard deviation.
2.5 Electrophysiological recordings in the thalamus
In order to quantify thalamic neuronal excitability, extracellular electrophysiological recordings were acquired in the VPL on day 7 after surgery [32]. Rats were anesthetized with sodium pentobarbital (45mg/kg, i.p.). A midline incision was made over the skull, and the soft tissue was resected to reveal the coronal, sagittal, and lambdoid cranial sutures. In order to gain access to the VPL, the cortex overlying the thalamus was exposed via an 8mm by 8mm craniotomy over the left hemisphere beginning at bregma and extending caudally and laterally [32]. The mid-cervical trachea was exposed, cannulated, and ventilated (CWE Inc.; Ardmore, PA) with room air at approximately 40 breaths/minute, and the end tidal CO2 concentration was monitored. The rat was mounted onto a stereotaxic frame with blunt ear bars, and the head was adjusted so bregma and lambda were in the horizontal plane. The dura was resected, and the brain was bathed in 37°C mineral oil. The core temperature of the rat was maintained at 35–37°C using a feedback-controlled heating plate with a rectal probe (Physitemp Instruments Inc.; Clifton, NJ). Anesthesia was maintained throughout all procedures with supplemental doses of sodium pentobarbital (5–10mg/kg, i.p.) as needed based on respiration and toe pinch reflexes.
Extracellular voltage potentials were recorded in the VPL using a glass-insulated tungsten electrode (FHC; Bowdoin, ME). Signals were processed with a 60Hz noise eliminator (HumBug, Quest Scientific; North Vancouver, Canada) and digitally sampled and stored at 25kHz (Micro1401, CED; Cambridge, England). Beginning at −2.5mm from bregma and 2.2mm left lateral, the electrode was lowered 5–7mm below the pial surface. Subsequent locations were probed at 0.2mm intervals in the anterior-posterior and medial-lateral planes, based on known coordinates and somatatopy of the rat VPL [50,51]. Neurons were identified by light brushing of the plantar surface of the right forepaw using a cotton swab. A stimulus train was applied to the forepaw consisting of ten light brushes, a subset of non-noxious and noxious von Frey filaments (1.4g, 4g, 10g, 26g), and a 10 second noxious pinch using a 60g vascular clip (WPI; Sarasota, FL) [14,32,52]. Each filament was applied for five consecutive 1-second stimulations. The different stimuli were applied at intervals of at least 60 seconds, and the number of evoked spikes was summed for each stimulus.
To analyze individual neurons, recordings during the stimulus train were spike-sorted using Spike2 (CED; Cambridge, England). Evoked spikes were summed over the continuous 10-second stimulus period for both the brush and pinch stimuli. The number of spikes evoked from the initial application of a von Frey filament until 1-second after the 5th application of the filament were counted for each neuron [5,14,32]. The duration of each stimulus was identified, and baseline firing was determined by counting the number of spikes over an equivalent time period (10 seconds) prior to each stimuli. Baseline spikes were subtracted from the total spike count for each stimulus in order to evaluate the evoked responses. Because the distribution of spike totals for each stimulus exhibited a positive skew, spike counts were log-transformed to obtain a normal distribution for statistical analyses. Evoked firing for each stimulus was compared between groups using a mixed-effect nested ANOVA with post hoc Tukey’s HSD test, since there was equal variance in the data from the different groups. As is conventional with reporting spike counts, data are presented as the mean±standard error of the mean.
2.6 Immunohistochemistry of VPL tissue
Brains were harvested at day 7 to measure expression of the glutamate transporters, EAAC1 and GLT-1, in the VPL (vehicle injury n=9, sham n=3, IB4-SAP injury n=12, IB4-SAP sham n=4). Brains from age-matched un-operated normal rats (n=2) were also included as controls. Rats were deeply anesthetized with sodium pentobarbital (65mg/kg) and transcardially perfused with phosphate-buffered saline and 4% paraformaldehyde. Samples were post-fixed in 4% paraformaldehyde overnight and stored in 30% sucrose for 6 days at 4°C. Whole brains were dissected coronally to include only the brain region (−2.28 to −2.64mm from bregma) containing the VPL. Samples were embedded, frozen, sectioned (16μm) and mounted onto slides. Sections were blocked for two hours at room temperature with 10% normal goat serum (Vector Labs; Burlingame, CA) and 0.3% Triton-X and incubated overnight at 4°C with primary antibodies for rabbit anti-GLT-1 (1:500; Abcam; Cambridge, MA) or rabbit anti-EAAC1 (1:500; Alpha Diagnostic; San Antonio, TX). Slides were washed with PBS, labeled with goat anti-rabbit Alexa-Fluor 568 secondary antibody (1:1000; Life Technologies; Carlsbad, CA) and cover-slipped using Fluorogel medium (EMS; Hatfield, PA). VPL sections were imaged at 200×, and the percent of GLT-1-postive and EAAC1-positive pixels within each image was quantified. The percent positive pixels in each group (reported as the mean±standard deviation) was normalized to normal values and compared via ANOVA with post-hoc Tukey’s HSD test, since there was equal variance in the data from the different groups.
3. Results
The biomechanical loading to the joint and facet capsule were the same for both groups of rats undergoing facet joint distraction (IB4-SAP and vehicle) when considering the metrics that capture the injury severity. The mean vertebral distractions (±standard deviation) for the IB4-SAP injury and vehicle injury groups were 0.39±0.15mm and 0.45±0.17mm, respectively. The mean capsule distraction (±standard deviation) was 0.22±0.09mm for the IB4-SAP injury group and was 0.19±0.08mm for the vehicle injury group. Maximum tensile strains (mean±standard deviation) were 17.34±11.43% and 13.57±10.19% for the IB4-SAP and vehicle injury groups, respectively. There is no difference in any of the mechanical metrics between the injury groups.
Injection of IB4-SAP prevented the development of forepaw mechanical hyperalgesia that is typically induced after painful facet joint injury. Overall, the response thresholds for the injury group with a vehicle injection were lower than the thresholds for both the IB4-SAP injury (p<0.00003; ANOVA main effect) and IB4-SAP sham (p<0.00003; ANOVA main effect) groups (Fig. 1). In fact, the thresholds for the IB4-SAP and IB4-sham groups were not different from their baseline pre-injury levels (Fig. 1). As early as 1 day after surgery, the response thresholds in the vehicle injury group were lower than both IB4-SAP injury (p<0.00001; post hoc) and IB4-SAP sham (p<0.023; post hoc) (Fig. 1). This difference persisted until day 7 when response thresholds for IB4-SAP injury (p<0.00001; post hoc) and IB4-SAP sham (p<0.0001; post hoc) remained significantly greater than the thresholds for the rats undergoing injury with vehicle injection (Fig. 1). The responses of the IB4-SAP injury group were not different from those receiving the IB4-SAP with sham surgery for any day (Fig. 1).
Fig. 1.
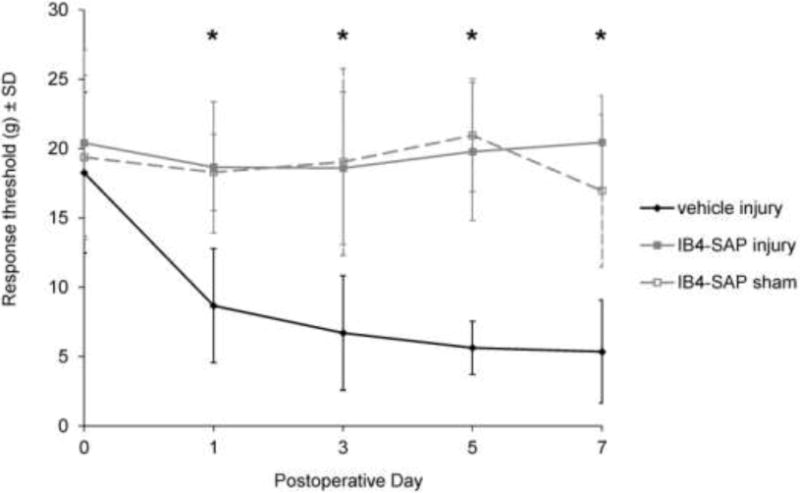
Forepaw mechanical hyperalgesia in response to von Frey filament stimulation. Injury with vehicle injection (vehicle injury) exhibits significantly reduced response thresholds starting at day 1 and persisting until day 7 compared to both IB4-SAP injury and IB4-SAP sham (*p<0.00003). IB4-SAP injury is not different from IB4-SAP sham on any day. Data are reported as the mean±standard deviation.
At day 7, facet joint injury induced hyperexcitability in thalamic neurons, which was attenuated by ablating non-peptidergic afferents in the facet joint (Fig. 2). A total of 135 neurons were recorded in the VPL at an average depth (±standard deviation) of 6.33±0.39mm, 2.65±0.69mm lateral, and −3.27±0.29mm caudal from bregma. The total number of evoked spikes was significantly increased following facet joint injury for paw stimulation with all von Frey magnitudes compared to the number of spikes evoked after the IB4-SAP injury (p<0.0054; post hoc), IB4-SAP sham (p<0.0037; post hoc), and sham (p<0.0170; post hoc) procedures (Fig. 2A). Evoked neuronal firing also increased after facet joint injury compared to all other groups for stimulation by both the brush (p<0.0020; post hoc) and the pinch (p<0.0097; post hoc) (Fig. 2B).
Fig. 2.
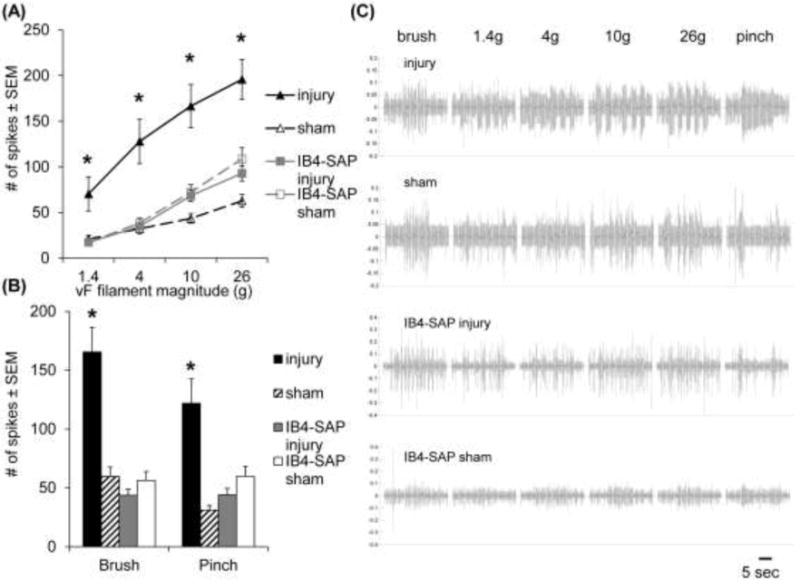
Neuronal hyperexcitability in the VPL of the thalamus at day 7. (A) Facet joint injury significantly increases the number of spikes evoked in the VPL in response to forepaw stimulation by 1.4, 4, 10, and 26g von Frey filaments, compared to all other groups (*p<0.017). (B) Neuronal hyperexcitability also increases after facet joint injury compared to all other groups (*p<0.0097) in response to both the brush and pinch stimuli. (C) Representative extracellular recordings during stimulation for each group; the scale bar represents 5 seconds. Data in (A) and (B) are reported as the mean±standard error of the mean.
Ablating IB4-positive afferents in the C6/C7 facet prevented the increased expression of GLT-1 and EAAC1 that is induced in the brain at day 7 after injury. Thalamic expression of the glutamate transporters, GLT-1 and EAAC1, at day 7 followed the behavioral response patterns (Figs. 1 & 3). After injury, GLT-1 expression in the VPL increased over sham (p<0.0198; post hoc), IB4-SAP sham (p<0.0001; post hoc), and normal (p<0.0001; post hoc) levels (Fig. 3B). GLT-1 reactivity in the vehicle injury group also was elevated over that observed in the IB4-SAP injury group (p<0.0072; post hoc) (Fig. 3B). Likewise, EAAC1 expression in the vehicle injury group also was significantly increased over all of the groups: sham (p<0.0001; post hoc), IB4-SAP injury (p<0.0001; post hoc), and IB4-SAP sham (p<0.0006; post hoc) and normal (p<0.0001; post hoc) (Fig. 3C). There were no differences between the IB4-SAP injury and IB4-SAP sham groups in the expression of either glutamate transporter in the VPL.
Fig. 3.
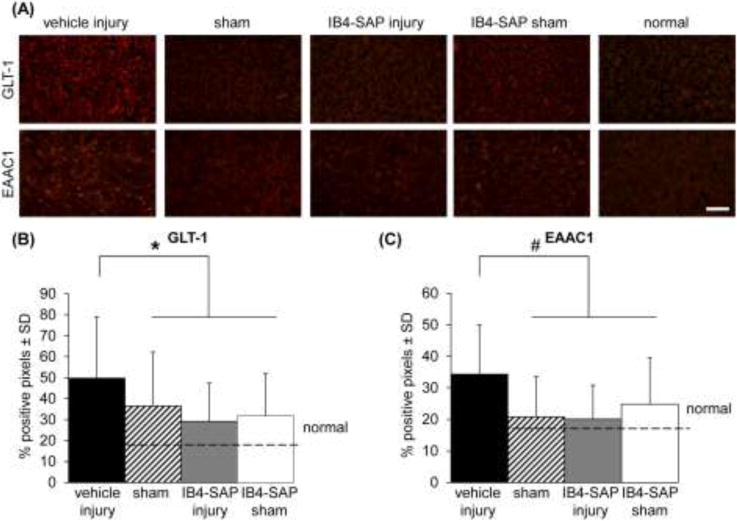
Representative images and quantification of GLT-1 and EAAC1 expression in the VPL of the thalamus at day 7. (A) Representative images of GLT-1 and EAAC1 expression in the VPL. The scale bar is 100μm and applies to all panels. (B) Thalamic expression of GLT-1 is significantly increased (*p<0.0198) after injury compared to all of the other groups: sham, IB4-SAP injury, and IB4-SAP sham and normal tissue. (C) Similarly, labeling of EAAC1 is elevated (#p<0.0006) following vehicle injury compared to all other groups. Data in (B) and (C) are reported as the mean±standard deviation.
4. Discussion
The non-peptidergic, IB4-positive neurons that innervate the cervical facet joint are critical for the establishment of pain and central sensitization after a mechanical joint injury. Eliminating these cells using the IB4-conjugated saporin prevents the development of the mechanical hyperalgesia that is typically present after facet joint injury (Fig. 1) and alters supraspinal neuronal and glutamatergic responses (Figs. 2 & 3). Together, these behavioral and thalamic data strongly suggest that input from non-peptidergic cells in the facet joint is requisite for initiating pain from its injury.
Glutamate is a major neurotransmitter in the central nervous system, and contributes to nociceptor sensitization, which has been shown to be an important regulator for maintaining behavioral hypersensitivity after a painful joint injury. In particular, the metabotropic glutamate receptor 5 (mGluR5) increases in models of peripheral joint pain [22,23,53]. Accordingly, the current findings suggest that removing IB4-positive joint afferents prior to a painful injury prevents any modification to glutamate transporter expression, and so, disrupts central sensitization in the thalamus (Figs. 2 & 3). Both GLT-1 and EAAC1 are upregulated in the VPL (Fig. 3). In contrast, spinal GLT-1 decreases in models of chronic pain [28,54,55] and glutamate levels in the VPL increase [56–58]. The elevated expression of glutamate transporters in the thalamus (Fig. 3) is consistent with an increase in glutamate in the brain. These results are also consistent with the increased hyperexcitability in the brain that is only evident after a painful facet joint injury (Fig. 2) since prolonged exposure to glutamate is correlated with increased neuronal hyperexcitability [59,60].
Although IB4-positive cells are critical components in the pain response, there is conflicting evidence for their mechanistic regulatory effects. Many studies report that ablating IB4-positive cells reduces pain [19,20,61,62]; but others assert that removing those cells can increase hyperalgesia, suggesting they may contribute to anti-nociceptive cascades [63,64]. Since ablating those afferents prevents the pain that is typically observed after the capsular ligament stretch used in this study (Fig. 1) the notion that IB4-positive cells have a role in nociception is supported. It is possible that the different behavioral findings reported in the literature after IB4-SAP administration could be attributed to differences in the testing methodology or the model used. In a study of formalin injection in the lip after ablating IB4-positive neurons in the trigeminal nerve, behavioral sensitivity was reported to increase [63]; in that work, face rubbing behavior was measured, which may capture different aspects of the pain experience. Moreover, in those studies showing increased sensitivity after ablation, nerves were injured either by chemical stimuli or underwent sustained constriction [63,64], both of which are largely neuropathic injuries. The joint pain model used here has been shown to have both neuropathic and inflammatory components [28,43,65].
Similar to the behavioral outcomes, removing non-peptidergic facet joint afferents prevents neuronal changes in the VPL that normally accompany facet joint pain (Fig. 2). Although spinal neuronal hyperexcitability is reported within 6 to 24 hours after this painful facet joint injury [14] and persists until day 7 [5,13], the current data are the first to show that a mechanical joint injury is sufficient to produce supraspinal hyperexcitability that is sustained (Fig. 2). Increased activation in the thalamus has been reported in arthritis and joint inflammation in both humans and animals [66–68]; patients with temporomandibular joint pain show functional and structural changes in the thalamus and somatosensory cortex (S1) [69]. Further, although deep brain stimulation in the thalamus offers relief in rats experiencing neuropathic pain [33], the mechanisms by which this is achieved are still unclear, and this technique has yet to be tested in joint pain. However, other brain regions, including the periaqueductal gray (PAG), anterior cingulate cortex (ACC), and primary somatosensory cortex (S1), are also implicated in joint pain [70–72], and a lesion to S1 alleviates neuronal hyperexcitability associated with inflammatory pain [73,74]. Examining if, and how, these other regions are affected by increased thalamic hyperexcitability from facet joint injury could provide further insight to pain perception and higher order sensory processing.
This study used IB4-SAP to identify the role of non-peptidergic joint afferents in mediating facet pain and supraspinal modifications. Although ablating non-peptidergic neurons in the cervical facet joint induced measurable changes in the thalamus, it is possible that other off-target changes occur in other brain regions or elsewhere in the peripheral and central nervous systems. Further work is necessary to evaluate other potential changes both local to, and remote from, the facet joint. Nevertheless, these findings suggest that non-peptidergic afferents innervating the facet joint are important for the development of sustained pain from mechanical joint injury. Ablating those cells using IB4-SAP in the facet joint prior to its painful injury prevented both neuronal hyperexcitability and glutamate transporter dysregulation in the thalamus. These findings indicate that non-peptidergic cells have widespread effects on joint pain and contribute to central sensitization. A better understanding of nociceptive pathways may help to develop more effective therapies for patients experiencing joint pain.
Highlights.
Ablating non-peptidergic afferents prevents hyperalgesia induced by facet injury.
Hyperexcitability in the VPL with facet pain is regulated by non-peptidergic afferents.
Thalamic glutamate transporter expression is altered by painful joint injury.
Non-peptidergic afferents contribute to the development of central sensitization.
Acknowledgments
This work was supported by the National Institutes of Health NIH/NIAMS [grant AR#056288]. The funding source had no involvement in the study design, data collection, analysis, or writing of this report.
Abbreviations
- GLT-1
glial glutamate transporter 1
- EAAC1
excitatory amino acid carrier 1
- IB4
isolectin-B4
- IB4-SAP
isolectin-B4 conjugated saporin
- VPL
ventral posterolateral nucleus
- PAG
periaqueductal gray
- ACC
anterior cingulate cortex
- S1
primary somatosensory cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
CL Weisshaar performed joint injections and surgeries, measured and analyzed mechanical hyperalgesia, and wrote the manuscript. JV Kras performed some of the surgeries and also analyzed some of the electrophysiology studies. PS Pall performed the electrophysiology studies and analyzed some of the electrophysiology data. S Kartha labeled and analyzed all of the brain tissue samples. BA Winkelstein obtained funding, designed the study, analyzed and interpreted the data, and wrote and edited the manuscript. All authors have approved the final submission.
References
- 1.Lord SM, Barnsley L, Wallis BJ, McDonald GJ, Bogduk N. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N Engl J Med. 1996;335:1721–6. doi: 10.1056/NEJM199612053352302. [DOI] [PubMed] [Google Scholar]
- 2.Pearson AM, Ivancic PC, Ito S, Panjabi MM. Facet joint kinematics and injury mechanisms during simulated whiplash. Spine (Phila Pa 1976) 2004;29:390–7. doi: 10.1097/01.brs.0000090836.50508.f7. http://www.ncbi.nlm.nih.gov/pubmed/15094535 (accessed April 7, 2016) [DOI] [PubMed] [Google Scholar]
- 3.Lu Y, Chen C, Kallakuri S, Patwardhan A, Cavanaugh JM. Neural response of cervical facet joint capsule to stretch: a study of whiplash pain mechanism. Stapp Car Crash J. 2005;49:49–65. doi: 10.4271/2005-22-0003. http://www.ncbi.nlm.nih.gov/pubmed/17096268 (accessed April 20, 2016) [DOI] [PubMed] [Google Scholar]
- 4.Lee KE, Davis MB, Mejilla RM, Winkelstein BA. In vivo cervical facet capsule distraction: mechanical implications for whiplash and neck pain. Stapp Car Crash J. 2004;48:373–395. doi: 10.4271/2004-22-0016. doi:2004-22-00016 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Quinn KP, Dong L, Golder FJ, Winkelstein BA. Neuronal hyperexcitability in the dorsal horn after painful facet joint injury. Pain. 2010;151:414–421. doi: 10.1016/j.pain.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kras JV, Tanaka K, Gilliland TM, Winkelstein BA. An anatomical and immunohistochemical characterization of afferents innervating the C6-C7 facet joint after painful joint loading in the rat. Spine (Phila Pa 1976) 2013;38:E325–31. doi: 10.1097/BRS.0b013e318285b5bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohtori S, Takahashi K, Moriya H. Calcitonin gene-related peptide immunoreactive DRG neurons innervating the cervical facet joints show phenotypic switch in cervical facet injury in rats. Eur Spine J. 2003;12:211–5. doi: 10.1007/s00586-002-0506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohtori S, Takahashi K, Chiba T, Yamagata M, Sameda H, Moriya H. Sensory innervation of the cervical facet joints in rats. Spine (Phila Pa 1976) 2001;26:147–50. doi: 10.1097/00007632-200101150-00007. http://www.ncbi.nlm.nih.gov/pubmed/11154533 (accessed April 7, 2016) [DOI] [PubMed] [Google Scholar]
- 9.Kallakuri S, Singh A, Chen C, Cavanaugh JM. Demonstration of substance P, calcitonin gene-related peptide, and protein gene product 9.5 containing nerve fibers in human cervical facet joint capsules. Spine (Phila Pa 1976) 2004;29:1182–6. doi: 10.1097/00007632-200406010-00005. http://www.ncbi.nlm.nih.gov/pubmed/15167655 (accessed July 14, 2016) [DOI] [PubMed] [Google Scholar]
- 10.Cavanaugh JM, Lu Y, Chen C, Kallakuri S. Pain generation in lumbar and cervical facet joints. J Bone Joint Surg Am. 2006;88(Suppl 2):63–7. doi: 10.2106/JBJS.E.01411. [DOI] [PubMed] [Google Scholar]
- 11.Kim JS, Ali MH, Wydra F, Li X, Hamilton JL, An HS, Cs-Szabo G, Andrews S, Moric M, Xiao G, Wang JHC, Chen D, Cavanaugh JM, Im HJ. Characterization of degenerative human facet joints and facet joint capsular tissues. Osteoarthr Cartil. 2015;23:2242–2251. doi: 10.1016/j.joca.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C, Lu Y, Kallakuri S, Patwardhan A, Cavanaugh JM. Distribution of A-delta and C-fiber receptors in the cervical facet joint capsule and their response to stretch. J Bone Joint Surg Am. 2006;88:1807–16. doi: 10.2106/JBJS.E.00880. [DOI] [PubMed] [Google Scholar]
- 13.Weisshaar CL, Winkelstein BA. Ablating spinal NK1-bearing neurons eliminates the development of pain and reduces spinal neuronal hyperexcitability and inflammation from mechanical joint injury in the rat. J Pain. 2014;15:378–86. doi: 10.1016/j.jpain.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crosby ND, Weisshaar CL, Winkelstein BA. Spinal neuronal plasticity is evident within 1 day after a painful cervical facet joint injury. Neurosci Lett. 2013;542:102–6. doi: 10.1016/j.neulet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kras JV, Weisshaar CL, Pall PS, Winkelstein BA. Pain from intra-articular NGF or joint injury in the rat requires contributions from peptidergic joint afferents. Neurosci Lett. 2015;604:193–8. doi: 10.1016/j.neulet.2015.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph EK, Levine JD. Hyperalgesic priming is restricted to isolectin B4-positive nociceptors. Neuroscience. 2010;169:431–5. doi: 10.1016/j.neuroscience.2010.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang X, Djouhri L, McMullan S, Berry C, Waxman SG, Okuse K, Lawson SN. Intense isolectin-B4 binding in rat dorsal root ganglion neurons distinguishes C-fiber nociceptors with broad action potentials and high Nav1.9 expression. J Neurosci. 2006;26:7281–92. doi: 10.1523/JNEUROSCI.1072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashiba H, Uchida Y, Senba E. Difference in binding by isolectin B4 to trkA and c-ret mRNA-expressing neurons in rat sensory ganglia. Mol Brain Res. 2001;95:18–26. doi: 10.1016/S0169-328X(01)00224-8. [DOI] [PubMed] [Google Scholar]
- 19.Tarpley JW, Kohler MG, Martin WJ. The behavioral and neuroanatomical effects of IB4-saporin treatment in rat models of nociceptive and neuropathic pain. Brain Res. 2004;1029:65–76. doi: 10.1016/j.brainres.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 20.Ye Y, Bae SS, Viet CT, Troob S, Bernabé D, Schmidt BL. IB4(+) and TRPV1(+) sensory neurons mediate pain but not proliferation in a mouse model of squamous cell carcinoma. Behav Brain Funct. 2014;10:5. doi: 10.1186/1744-9081-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarez P, Chen X, Bogen O, Green PG, Levine JD. IB4(+) nociceptors mediate persistent muscle pain induced by GDNF. J Neurophysiol. 2012;108:2545–53. doi: 10.1152/jn.00576.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisshaar CL, Dong L, Bowman AS, Perez FM, Guarino BB, Sweitzer SM, Winkelstein BA. Metabotropic glutamate receptor-5 and protein kinase C-epsilon increase in dorsal root ganglion neurons and spinal glial activation in an adolescent rat model of painful neck injury. J Neurotrauma. 2010;27:2261–2271. doi: 10.1089/neu.2010.1460. [DOI] [PubMed] [Google Scholar]
- 23.Dong L, Quindlen JC, Lipschutz DE, Winkelstein BA. Whiplash-like facet joint loading initiates glutamatergic responses in the DRG and spinal cord associated with behavioral hypersensitivity. Brain Res. 2012;1461:51–63. doi: 10.1016/j.brainres.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A Novel Nociceptor Signaling Pathway Revealed in Protein Kinase C ε Mutant Mice. Neuron. 1999;24:253–260. doi: 10.1016/S0896-6273(00)80837-5. [DOI] [PubMed] [Google Scholar]
- 25.Sweitzer SM, Wong SME, Peters MC, Mochly-Rosen D, Yeomans DC, Kendig JJ. Protein kinase C epsilon and gamma: involvement in formalin-induced nociception in neonatal rats. J Pharmacol Exp Ther. 2004;309:616–25. doi: 10.1124/jpet.103.060350. [DOI] [PubMed] [Google Scholar]
- 26.Lourenço Neto F, Schadrack J, Platzer S, Zieglgänsberger W, Tölle TR, Castro-Lopes JM. Expression of metabotropic glutamate receptors mRNA in the thalamus and brainstem of monoarthritic rats. Mol Brain Res. 2000;81:140–154. doi: 10.1016/S0169-328X(00)00176-5. [DOI] [PubMed] [Google Scholar]
- 27.Dong L, Winkelstein BA. Simulated whiplash modulates expression of the glutamatergic system in the spinal cord dynamic cervical facet loading. J Neurotrauma. 2010;27:163–174. doi: 10.1089/neu.2009.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crosby ND, Gilliland TM, Winkelstein BA. Early afferent activity from the facet joint after painful trauma to its capsule potentiates neuronal excitability and glutamate signaling in the spinal cord. Pain. 2014;155:1878–87. doi: 10.1016/j.pain.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dostrovsky JO. Role of thalamus in pain. Prog Brain Res. 2000;129:245–57. doi: 10.1016/S0079-6123(00)29018-3. [DOI] [PubMed] [Google Scholar]
- 31.Huh Y, Bhatt R, Jung D, Shin H, Cho J. Interactive responses of a thalamic neuron to formalin induced lasting pain in behaving mice. PLoS One. 2012;7:e30699. doi: 10.1371/journal.pone.0030699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Syré PP, Weisshaar CL, Winkelstein BA. Sustained neuronal hyperexcitability is evident in the thalamus after a transient cervical radicular injury. Spine (Phila Pa 1976) 2014;39:E870–E877. doi: 10.1097/BRS.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 33.Iwata M, LeBlanc BW, Kadasi LM, Zerah ML, Cosgrove RG, Saab CY. High-frequency stimulation in the ventral posterolateral thalamus reverses electrophysiologic changes and hyperalgesia in a rat model of peripheral neuropathic pain. Pain. 2011;152:2505–13. doi: 10.1016/j.pain.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Ita M, Crosby N, Bulka B, Winkelstein B. Painful cervical facet joint injury is accompanied by changes in the number of excitatory and inhibitory synapses in the superficial dorsal horn that deferentially relate to local tissue injury severity. Spine (Phila Pa 1976) 2016 doi: 10.1097/BRS.0000000000001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crosby ND, Zaucke F, Kras JV, Dong L, Luo ZD, Winkelstein BA. Thrombospondin-4 and excitatory synaptogenesis promote spinal sensitization after painful mechanical joint injury. Exp Neurol. 2015;264:111–120. doi: 10.1016/j.expneurol.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou XF, Chie ET, Deng YS, Zhong JH, Xue Q, Rush RA, Xian CJ. Injured primary sensory neurons switch phenotype for brain-derived neurotrophic factor in the rat. Neuroscience. 1999;92:841–853. doi: 10.1016/S0306-4522(99)00027-5. [DOI] [PubMed] [Google Scholar]
- 37.Winkelstein BA, Kras JV. Is there an antinociceptive role for peripheral brain-derived neurotrophic factor? Spine J. 2010;10:733–735. doi: 10.1016/j.spinee.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Han Y, Li N, Zeiler SR, Pelled G. Peripheral nerve injury induces immediate increases in layer v neuronal activity. Neurorehabil Neural Repair. 2013;27:664–72. doi: 10.1177/1545968313484811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.St-Jacques B, Ma W. Peripheral prostaglandin E2 prolongs the sensitization of nociceptive dorsal root ganglion neurons possibly by facilitating the synthesis and anterograde axonal trafficking of EP4 receptors. Exp Neurol. 2014;261:354–66. doi: 10.1016/j.expneurol.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 40.Dong L, Guarino BB, Jordan-Sciutto KL, Winkelstein BA. Activating transcription factor 4, a mediator of the integrated stress response, is increased in the dorsal root ganglia following painful facet joint distraction. Neuroscience. 2011;193:377–386. doi: 10.1016/j.neuroscience.2011.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kras JV, Weisshaar CL, Quindlen J, Winkelstein BA. Brain-derived neurotrophic factor is upregulated in the cervical dorsal root ganglia and spinal cord and contributes to the maintenance of pain from facet joint injury in the rat. J Neurosci Res. 2013;91:1312–1321. doi: 10.1002/jnr.23254. [DOI] [PubMed] [Google Scholar]
- 42.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 43.Dong L, Smith JR, Winkelstein BA. Ketorolac reduces spinal astrocytic activation and PAR1 expression associated with attenuation of pain after facet joint injury. J Neurotrauma. 2013;30:818–825. doi: 10.1089/neu.2012.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2010;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, Bardoni R. BDNF as a pain modulator. Prog Neurobiol. 2008;85:297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Hucho TB, Dina OA, Levine JD. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. J Neurosci. 2005;25:6119–6126. doi: 10.1523/JNEUROSCI.0285-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiley RG, Kline RH, Vierck CJ. Anti-nociceptive effects of selectively destroying substance P receptor-expressing dorsal horn neurons using [Sar9,Met(O2)11]-substance P-saporin: behavioral and anatomical analyses. Neuroscience. 2007;146:1333–45. doi: 10.1016/j.neuroscience.2007.01.066. [DOI] [PubMed] [Google Scholar]
- 48.Lee KE, Winkelstein BA. Joint distraction magnitude is associated with different behavioral outcomes and substance P levels for cervical facet joint loading in the rat. J Pain. 2009;10:436–445. doi: 10.1016/j.jpain.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 50.Francis JT, Xu S, Chapin JK. Proprioceptive and cutaneous representations in the rat ventral posterolateral thalamus. J Neurophysiol. 2008;99:2291–2304. doi: 10.1152/jn.01206.2007. [DOI] [PubMed] [Google Scholar]
- 51.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th. Academic Press; San Deigo, CA: 2007. [Google Scholar]
- 52.Dong L, Crosby ND, Winkelstein BA. Gabapentin alleviates facet-mediated pain in the rat through reduced neuronal hyperexcitability and astrocytic activation in the spinal cord. J Pain. 2013;14:1564–72. doi: 10.1016/j.jpain.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li B, Lu L, Tan X, Zhong M, Guo Y, Yi X. Peripheral Metabotropic Glutamate Receptor Subtype 5 Contributes to Inflammation-Induced Hypersensitivity of the Rat Temporomandibular Joint. J Mol Neurosci. 2013;51:710–718. doi: 10.1007/s12031-013-0052-2. [DOI] [PubMed] [Google Scholar]
- 54.Nicholson KJ, Gilliland TM, Winkelstein BA. Upregulation of GLT-1 by treatment with ceftriaxone alleviates radicular pain by reducing spinal astrocyte activation and neuronal hyperexcitability. J Neurosci Res. 2014;92:116–129. doi: 10.1002/jnr.23295. [DOI] [PubMed] [Google Scholar]
- 55.Ramos KM, Lewis MT, Morgan KN, Crysdale NY, Kroll JL, Taylor FR, Harrison JA, Sloane EM, Maier SF, Watkins LR. Spinal upregulation of glutamate transporter GLT-1 by ceftriaxone: therapeutic efficacy in a range of experimental nervous system disorders. Neuroscience. 2010;169:1888–900. doi: 10.1016/j.neuroscience.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naderi A, Asgari AR, Zahed R, Ghanbari A, Samandari R, Jorjani M. Estradiol attenuates spinal cord injury-related central pain by decreasing glutamate levels in thalamic VPL nucleus in male rats. Metab Brain Dis. 2014;29:763–70. doi: 10.1007/s11011-014-9570-z. [DOI] [PubMed] [Google Scholar]
- 57.Silva E, Quiñones B, Freund N, Gonzalez LE, Hernandez L. Extracellular glutamate, aspartate and arginine increase in the ventral posterolateral thalamic nucleus during nociceptive stimulation. Brain Res. 2001;923:45–9. doi: 10.1016/s0006-8993(01)03195-x. http://www.ncbi.nlm.nih.gov/pubmed/11743971 (accessed April 13, 2016) [DOI] [PubMed] [Google Scholar]
- 58.Abarca C, Silva E, Sepúlveda MJ, Oliva P, Contreras E. Neurochemical changes after morphine, dizocilpine or riluzole in the ventral posterolateral thalamic nuclei of rats with hyperalgesia. Eur J Pharmacol. 2000;403:67–74. doi: 10.1016/S0014-2999(00)00502-1. [DOI] [PubMed] [Google Scholar]
- 59.Gwak YS, Hulsebosch CE. Neuronal hyperexcitability: a substrate for central neuropathic pain after spinal cord injury. Curr Pain Headache Rep. 2011;15:215–22. doi: 10.1007/s11916-011-0186-2. [DOI] [PubMed] [Google Scholar]
- 60.Ghanbari A, Asgari AR, Kaka GR, Falahatpishe HR, Naderi A, Jorjani M. In vivo microdialysis of glutamate in ventroposterolateral nucleus of thalamus following electrolytic lesion of spinothalamic tract in rats. Exp Brain Res. 2014;232:415–21. doi: 10.1007/s00221-013-3749-0. [DOI] [PubMed] [Google Scholar]
- 61.Vulchanova L, Olson TH, Stone LS, Riedl MS, Elde R, Honda CN. Cytotoxic targeting of isolectin IB4-binding sensory neurons. Neuroscience. 2001;108:143–55. doi: 10.1016/s0306-4522(01)00377-3. http://www.ncbi.nlm.nih.gov/pubmed/11738138 (accessed April 14, 2016) [DOI] [PubMed] [Google Scholar]
- 62.Alvarez P, Gear RW, Green PG, Levine JD. IB4-saporin attenuates acute and eliminates chronic muscle pain in the rat. Exp Neurol. 2012;233:859–65. doi: 10.1016/j.expneurol.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oyamaguchi A, Abe T, Sugiyo S, Niwa H, Takemura M. Selective elimination of isolectin B4-binding trigeminal neurons enhanced formalin-induced nocifensive behavior in the upper lip of rats and c-Fos expression in the trigeminal subnucleus caudalis. Neurosci Res. 2016;103:40–7. doi: 10.1016/j.neures.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 64.Taylor AMW, Osikowicz M, Ribeiro-da-Silva A. Consequences of the ablation of nonpeptidergic afferents in an animal model of trigeminal neuropathic pain. Pain. 2012;153:1311–9. doi: 10.1016/j.pain.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 65.Kras JV, Dong L, Winkelstein BA. Increased interleukin-1α and prostaglandin E2 expression in the spinal cord at 1 day after painful facet joint injury: evidence of early spinal inflammation. Spine (Phila Pa 1976) 2014;39:207–12. doi: 10.1097/BRS.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sofat N, Smee C, Hermansson M, Howard M, Baker EH, Howe FA, Barrick TR. Functional MRI demonstrates pain perception in hand osteoarthritis has features of central pain processing. J Biomed Graph Comput. 2013;3 doi: 10.5430/jbgc.v3n4p20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neto FL, Carvalhosa AR, Ferreira-Gomes J, Reguenga C, Castro-Lopes JM. Delta opioid receptor mRNA expression is changed in the thalamus and brainstem of monoarthritic rats. J Chem Neuroanat. 2008;36:122–7. doi: 10.1016/j.jchemneu.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 68.Hutchison WD, Lühn MA, Schmidt RF. Responses of lateral thalamic neurons to algesic chemical stimulation of the cat knee joint. Exp Brain Res. 1994;101:452–64. doi: 10.1007/BF00227338. http://www.ncbi.nlm.nih.gov/pubmed/7851512 (accessed April 13, 2016) [DOI] [PubMed] [Google Scholar]
- 69.Lin CS. Brain signature of chronic orofacial pain: a systematic review and meta-analysis on neuroimaging research of trigeminal neuropathic pain and temporomandibular joint disorders. PLoS One. 2014;9:e94300. doi: 10.1371/journal.pone.0094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DeSantana JM, Da Silva LFS, De Resende MA, Sluka KA. Transcutaneous electrical nerve stimulation at both high and low frequencies activates ventrolateral periaqueductal grey to decrease mechanical hyperalgesia in arthritic rats. Neuroscience. 2009;163:1233–41. doi: 10.1016/j.neuroscience.2009.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lamour Y, Guilbaud G, Willer JC. Altered properties and laminar distribution of neuronal responses to peripheral stimulation in the SmI cortex of the arthritic rat. Brain Res. 1983;273:183–7. doi: 10.1016/0006-8993(83)91111-3. http://www.ncbi.nlm.nih.gov/pubmed/6616226 (accessed April 18, 2016) [DOI] [PubMed] [Google Scholar]
- 72.Guo JD, Wang H, Zhang YQ, Zhao ZQ. Alterations of membrane properties and effects of D-serine on NMDA-induced current in rat anterior cingulate cortex neurons after monoarthritis. Neurosci Lett. 2005;384:245–9. doi: 10.1016/j.neulet.2005.04.096. [DOI] [PubMed] [Google Scholar]
- 73.Uhelski ML, Davis MA, Fuchs PN. Pain affect in the absence of pain sensation: evidence of asomaesthesia after somatosensory cortex lesions in the rat. Pain. 2012;153:885–92. doi: 10.1016/j.pain.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 74.Guilbaud G, Benoist JM, Condes-Lara M, Gautron M. Further evidence for the involvement of SmI cortical neurons in nociception: their responsiveness at 24 hr after carrageenin-induced hyperalgesic inflammation in the rat. Somatosens Mot Res. 1993;10:229–44. doi: 10.3109/08990229309028834. http://www.ncbi.nlm.nih.gov/pubmed/8237212 (accessed April 18, 2016) [DOI] [PubMed] [Google Scholar]


