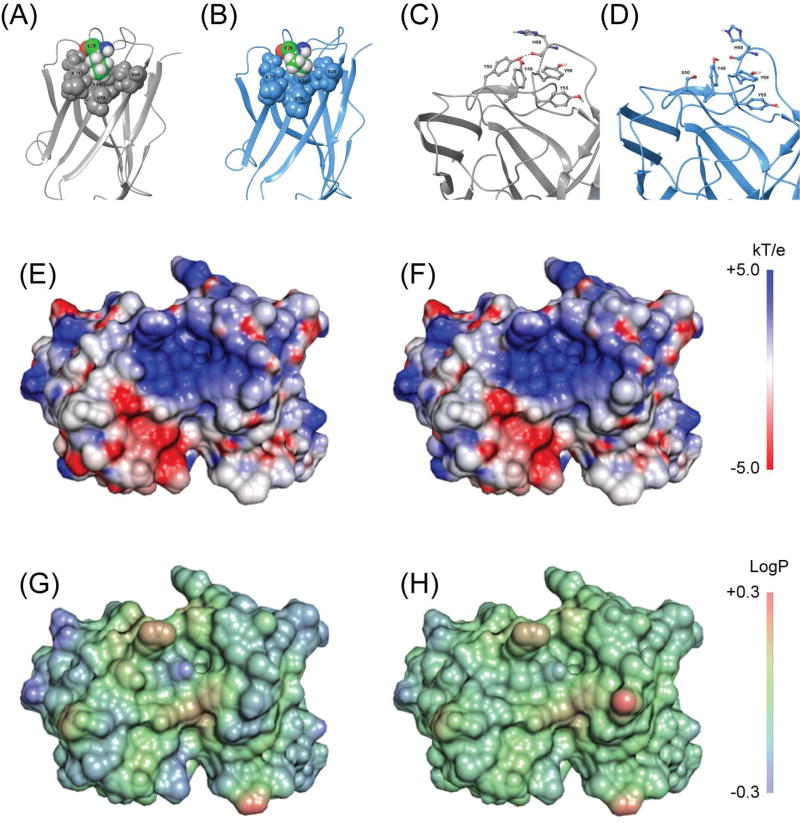
Figure 6.
(A–D) Snapshots of residues identified as contributing to antibody flexibility. (A) In the germline structure, L29 of the heavy chain (shown in CPK representation with green carbons) is involved in van der Waals packing with heavy chain residues V34, V78, and K71. (B) In the mature structure, L29 is mutated to V29. The result is a destabilization of the HC loop by a decrease in van der Waals packing. (C) In the germline light chain, Y50 hydrogen bonds to the backbone carbonyl of HC H98, and forms a multiple ring-stacking interaction with LC Y49, LC Y55, and HC Y99. (D) The mutation in the mature structure to LC S50 weakens these interactions. (E–H) Top-down view of the antigen binding site surface of 3F8. Electrostatic surface of (E) germline and (F) affinity mature 3F8 structures. Surface hydrophobicity of (G) germline and (H) affinity mature 3F8 structures. Surfaces were calculated using VASCo and DelPhi and rendered in PyMOL.