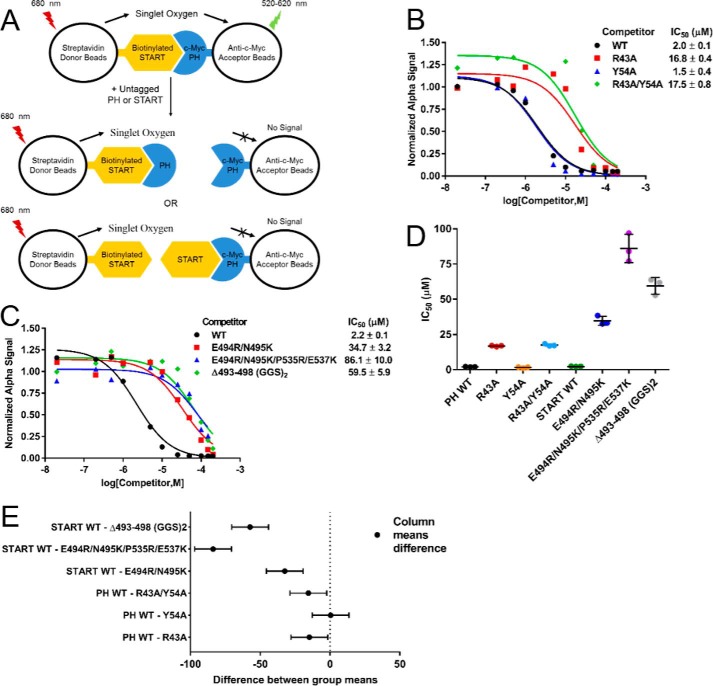
Figure 4.
CERT START domain β6′/β7′ and β8′/β9′ loops interact with PH through its PtdIns(4)P-binding site. The PH–START interface was evaluated by an AlphaScreen competition assay. A, design of the AlphaScreen competition assay. Biotinylated START and c-Myc PH domain are immobilized on streptavidin-coated donor beads and anti-c-Myc-coated acceptor beads, respectively. Illumination of donor beads produces singlet oxygen. Interaction between the tagged PH and START domains brings donor and acceptor beads into close proximity, allowing energy from singlet oxygen to be transferred to the acceptor beads and produce a light signal. The addition of untagged competitor proteins reduces the signal. B, competition for binding to biotinylated START domain by untagged PH WT and PH domain mutants. C, competition for binding to c-Myc PH domain by untagged START WT and START domain mutants. Each competitor was tested in triplicate with a representative data set shown. Data analysis and curve-fitting parameters are found under “Experimental Procedures.” Legends for panels B and C are inset. D, mean and S.D. for the IC50 values measured in B and C. E, one-way analysis of the IC50 values measured in B and C. Shown are the Sidak 99% confidence intervals for the Sidak's multiple comparisons tests.