Figure 2.
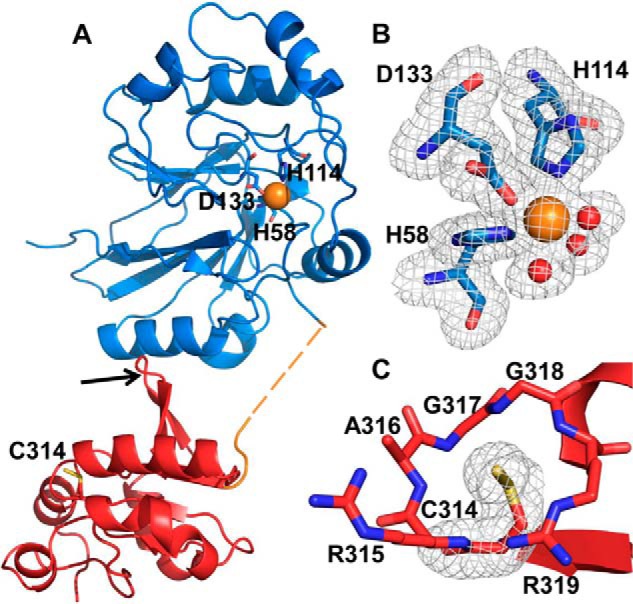
Crystal structure of BpPRF. The X-ray crystal structure of ΔCBpPRF was solved at 1.79 Å resolution by molecular replacement using the AaPRF structure (PDB code 3TP9) as a template. A, structure of the BpPRF monomer consists of an N-terminal PDO domain (blue), a 15-residue linker region (orange), and a C-terminal rhodanese domain (red). The linker region lacked density for eight residues (dashed lines) suggesting a flexible, disordered state. The iron (orange sphere) ligands in the PDO domain and the active-site cysteine, Cys-314, in the rhodanese domain are shown in stick representation. The rhodanese domain β-hairpin extension is highlighted by the red arrow. B, close-up of the PDO-active site with representative electron density (3.0σ Fo − Fc omit density; gray mesh) for side chains of His-58, His-114, Asp-133, and three water molecules (shown as red spheres) coordinated to the iron center (orange sphere). C, close-up of the rhodanese domain active site with representative electron density (3.0σ Fo − Fc omit density; gray mesh) for the Cys-314 side chain displaying the additional density for the persulfide modification.
