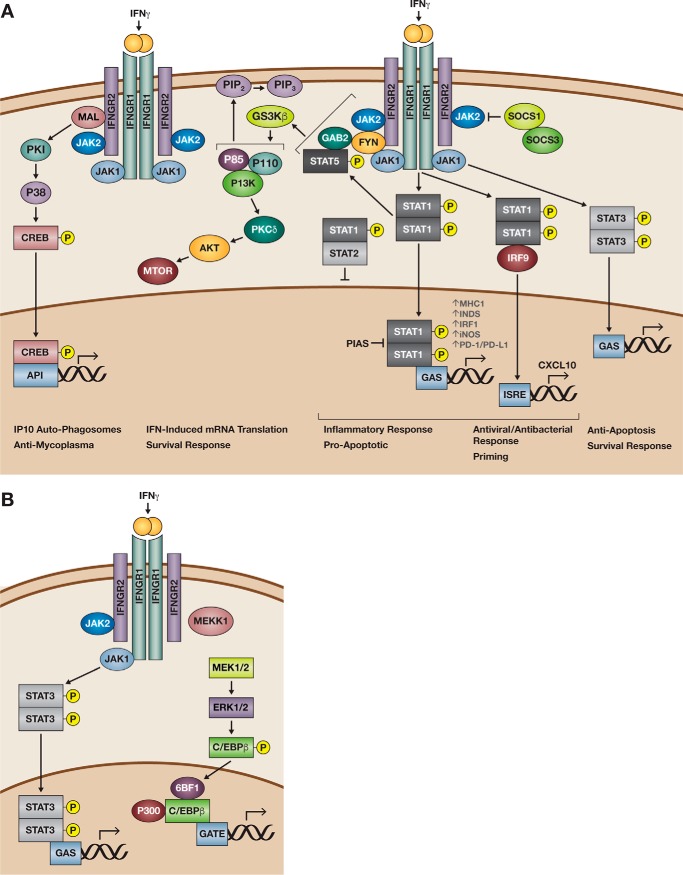
Figure 1.
Overview of the STAT-1 canonical and non-canonical signaling pathways elicited by IFNγ. As a dimer, IFNγ (orange) binds the IFNGR, which is composed of the IFNGR1 and IFNGR2 subunits, the kinases Jak1/Jak2. A, in canonical IFNγ signaling, phosphorylation of Jak1 and JAK2 results in the phosphorylation of STAT1 (center). A STAT1 homodimer translocates to the nucleus and binds to GAS found in the promoters of IFNγ-regulated genes such as HLA-A, NOS-2, IRF1, PDCD1, and CD274. Recruitment of adaptor proteins associated with IFNGR2 such as MAL and Fyn results in non-canonical STAT1 signaling. MAL-dependent IFN-γ receptor (IFNGR) signaling elicits signaling via MAPK p38 phosphorylation to up-regulate expression of the chemokine IP-10, antimycoplasma proteins, and formation of autophagosomes (left). GSK3β activation and Fyn elicit pSTAT5 recruitment to activate PI3K to regulate cell membrane permeabilization. Alternatively, IFNγ activation of GSK3β via PKCδ activates AKT/mTOR regulation of survival responses. Nevertheless, upon STAT activation, control mechanisms aiming to regulate signaling target either the JAK catalytic sites with SOCS proteins (upper right corner) or blockade of the STAT dimers binding to GAS sites with PIAS or through binding with un-phosphorylated STAT2. Alternatively, IFN priming increases IRF9, which is recruitment to STAT1 dimers for binding to ISRE sites. Antiviral and antibacterial responses benefit from this mechanism. B, in cells not expressing STAT1, STAT3 can be phosphorylated by Jak1/Jak2 resulting in translocation of the STAT3 dimer to GAS sites. Moreover, IFNγ activation of ERK results in C/EBPβ activation and binding to a novel IFN-response element (GATE). Figure has been adapted and modified from Refs. 17 and 29.