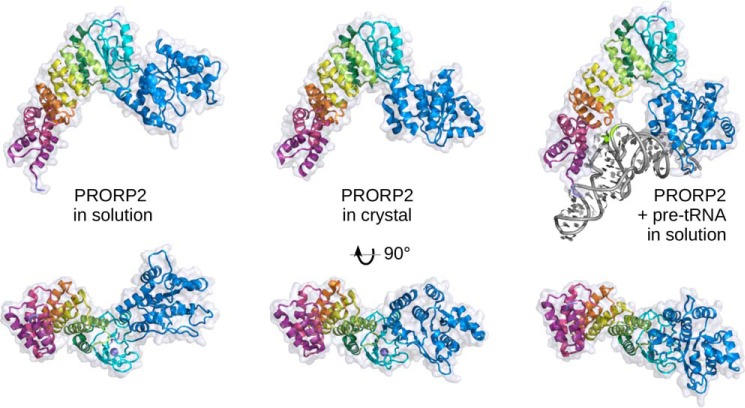
Figure 5.
Domain reorientation in PRORP2. A comparison of structures of PRORP2 in solution, in the crystal, and in solution in complex with a pre-tRNA substrate (left, middle, and right, respectively) highlights the movement of the catalytic domain with respect to the PPR domain and the zinc-binding domain. The three models have been superimposed according to the latter two domains and are shown in two perpendicular views, from the side and from the inner region of the Λ-shape facing the RNA substrate. In solution, the catalytic domain free and complexed with PRORP2 undergoes a 52° and 23° rotation compared with its position in the crystal structure, illustrating the flexibility of the hinge region and the structural plasticity of PRORP2. The characterization of PRORP1 in solution (see supplemental Fig. S4) shows a similar behavior and suggests that this may be a general property of PRORP enzymes.