FIG 1.
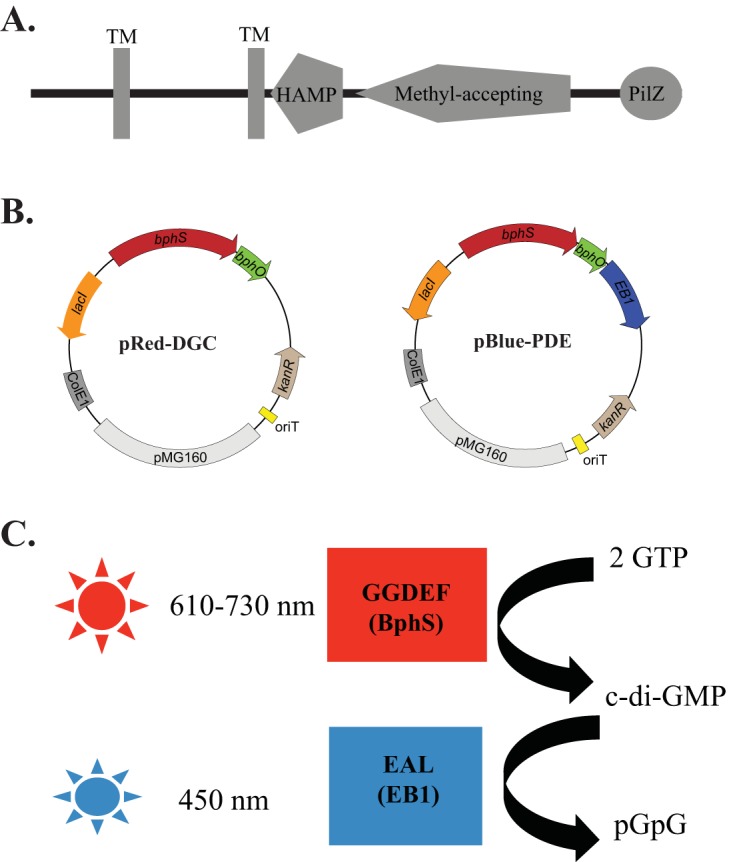
Domain architecture of Tlp1 and dichromatic optogenetic system for manipulating c-di-GMP levels in proteobacteria. (A) Tlp1 is a prototypical chemotaxis receptor comprising an N-terminal sensing domain between two transmembrane (TM) regions exposed to the periplasm followed by a HAMP (histidine kinase, adenyl cyclase, methyl-accepting protein, and phosphatase) domain and a C-terminal signaling methyl-accepting (MA) domain. A PilZ domain is also present at the extreme C terminus of Tlp1. (B) Maps of plasmids pRed-DGC and pBlue-PDE, with pRed-DGC expressing the red-light-activated diguanylate cyclase BphS and pBlue-PDE expressing the blue-light-activated phosphodiesterase EB1. (C) Wavelengths of light used to activate BphS and EB1 activities in order to induce c-di-GMP synthesis and degradation, respectively.
