Figure 2. Standard molecular orbital electronic configurations of the outer electrons of relevant divalent metal di-cations for the first four rows of the period table.
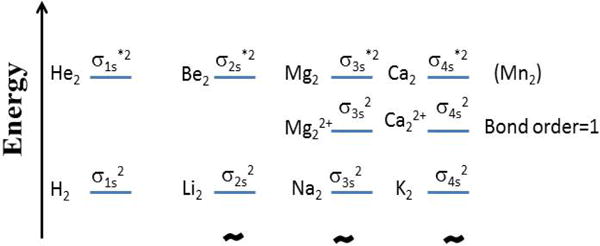
Energy levels and their spacing adjusted for readability. The “*” superscript of the orbital label designates antibonding. He2, Be2, Mg2 and Ca2 (Mn2) have a net formal bond order of zero, whereas their di-cations have a net formal bond order of 1.
