Abstract
Recessive dystrophic epidermolysis bullosa (RDEB) is a rare monogenic blistering disorder caused by lack of functional type VII collagen, leading to skin fragility and subsequent trauma-induced separation of the epidermis from the underlying dermis. 46% of RDEB patients harbor at least one premature termination codon (PTC) mutation in COL7A1 and previous studies have shown that aminoglycosides are able to overcome RDEB PTC mutations by inducing “read-through” and incorporation of an amino acid at the PTC site. However, aminoglycoside toxicity will likely prevent widespread clinical application. Here the FDA-approved drug amlexanox was tested for its ability to read-through PTC mutations in RDEB patient derived cells. Eight of 12 different PTC alleles responded to treatment and produced full length protein, in some cases over 50% relative to normal controls. Read-through type VII collagen was readily detectable in cell culture media and also localized to the dermal-epidermal junction in organotypic skin culture. Amlexanox increased COL7A1 transcript and the phosphorylation of UPF-1, an RNA helicase associated with nonsense mediated mRNA decay (NMD), suggesting that amlexanox inhibits NMD in RDEB patient cells that read-through. This pre-clinical study demonstrates the potential of re-purposing amlexanox for treatment of RDEB patients harboring PTC mutation in COL7A1.
Keywords: recessive dystrophic epidermolysis bullosa, premature termination codon, amlexanox, read-through, type VII collagen
INTRODUCTION
Epidermolysis bullosa (EB) is a group of inherited blistering diseases caused by mutations in genes encoding proteins critical for skin integrity (Fine et al., 2014). Dystrophic EB is caused by mutations in COL7A1 leading to defective type VII collagen, a protein that localizes to the basement membrane zone (BMZ) and provides adhesion of the epidermis to the dermis through interaction with collagen IV, laminin 332, and collagen I (Brittingham et al., 2006; Chen et al., 1999; Villone et al., 2008). Both keratinocytes and fibroblasts synthesize and secrete type VII collagen which, after cleavage of the non-collagenous carboxy-terminal NC2 domain, assembles in antiparallel tail-to-tail dimers which subsequently aggregate to form anchoring fibrils (Bruckner-Tuderman et al., 1995; Chung and Uitto, 2010). The proper assembly of anchoring fibrils is required for stable attachment between the epidermis and dermis and inability to form anchoring fibrils due to genetic mutation in COL7A1 results in skin fragility, trauma-induced blistering followed by chronic wounds with poor healing and excessive scarring. The recessive form of dystrophic EB (recessive dystrophic EB, RDEB; OMIM #226600) is often a devastating disorder, leading to extensive skin blistering, nail dystrophy, alopecia, and mitten deformities of the hands and feet. Extracutaneous involvement of esophageal and other mucosal epithelium further complicates this disease (Fine et al., 2014; Fine and Mellerio, 2009) and RDEB patients suffer from excessive fibrosis at the sites of tissue damage associated with development of aggressive and frequently fatal squamous cell carcinomas (Fine et al., 2009; Mittapalli et al., 2016; Ng et al., 2012). A correlation exists between the amount of type VII collagen present at the BMZ and the severity of the disease (McGrath et al., 1993). Premature termination codon mutations (PTCs) in COL7A1 are reported in about 46% of RDEB patients (van den Akker et al., 2011; Wertheim-Tysarowska et al., 2012), and those individuals harboring two PTC mutations (around 10% of cases reported) express very low amounts, if any, of truncated type VII collagen and have the most severe subtype of the disease - RDEB-severe generalized (Fine et al., 2014).
Significant progress toward development of therapeutic approaches for RDEB has been made in recent years (Uitto et al., 2016). Treatment strategies that have shown promising pre-clinical data include bone marrow stem cell transplantation (Liao et al., 2015; Tamai et al., 2011; Tolar et al., 2009), viral delivery of COL7A1 to correct expression ex-vivo (Chen et al., 2002; Jackow et al., 2016; Siprashvili et al., 2010), and protein therapy (Woodley et al., 2013). However, those approaches that have translated into the clinic are not without complications - in some cases they result in significant mortality (Wagner et al., 2010) or involve long and expensive procedures dependent on specialized equipment and infrastructure (Siprashvili et al., 2016). More recently, RNA editing approaches have shown preclinical promise (Bremer et al., 2016; Goto et al., 2006; Titeux et al., 2010; Turczynski et al., 2016), and gene correction by read-through of PTC mutations has been demonstrated in vitro (Cogan et al., 2014). In the latter study, read-through of COL7A1 PTC mutations by aminoglycosides demonstrated efficient expression of full length type VII collagen protein in patient cells with homozygous PTC mutations. However, systemic long term administration of aminoglycosides, such as gentamicin, can be associated with toxicity, mainly ototoxity and kidney failure (Tokgoz et al., 2010; Walker and Shah, 1987), which will ultimately slow down translation of this drug into clinical practice.
In this study, read-through efficacy of amlexanox, an FDA approved drug previously reported to induce read-through in human cells (Gonzalez-Hilarion et al., 2012), was evaluated in RDEB patient cells in vitro. Amlexanox was initially approved for the treatment of mouth ulcers and is currently in Phase II clinical trials for diabetes mellitus type II (www.clinicaltrials.gov). Here pre-clinical evidence is presented supporting amelxanox for treatment of RDEB patients harboring PTC mutation in COL7A1.
RESULTS
Amlexanox induces full length type VII collagen synthesis in RDEB fibroblasts and keratinocytes with COL7A1 PTC mutation
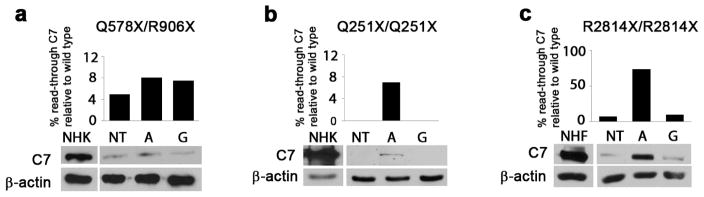
Previous reports have shown that amlexanox is well tolerated by cultured cells, showing little toxicity up to 125 μM concentrations (Gonzalez-Hilarion et al., 2012). In agreement with these previous studies, incubation of skin fibroblasts and keratinocytes with amlexanox showed little toxicity up to 250 μM, although growth was retarded over 48 hours at higher concentrations (Figure S1 and data not shown). Next, RDEB patient cells harboring homozygous COL7A1 PTC mutation (p.Q251X) were incubated with a range of amlexanox concentrations, and 250 μM was identified as the concentration inducing greatest level of full length type VII collagen expression after 48 hours (Figure S2). This dose was used for all subsequent experiments. Amlexanox and gentamicin were then assessed for read-through in cells derived from 14 RDEB patients harboring 12 different PTC mutations. After 48 hours of treatment with amlexanox, increased full length type VII collagen (290 kDa) was observed by Western blot analysis of total cell lysates from RDEB keratinocytes and fibroblasts isolated from eight RDEB patients. In total, four out of 12 PTC mutations did not show evidence of read-through (Table 1) either with amlexanox or with gentamicin. In those samples with homozygous PTC mutations that responded to treatment, a higher efficacy of read-through with amlexanox, compared to gentamicin was observed (Figure 1). In cells with compound heterozygous PTC/missense mutations (p.G2073D/p.R578X) a clear increase in type VII collagen in total cell lysate was not apparent due to the large amount of intracellular protein as result of the presence of the missense mutation (Figure 2a). Overall, read-through induced either by amlexanox or by gentamicin was only observed in those patient cells which expressed detectable levels of full length type VII collagen, albeit at considerably lower levels and presumably as a result of endogenous read-through (Figure 1).
Table 1.
Cells used in this study. The table shows the name of cell lines, their mutations, immortalization conditions, the nucleotide sequence of the stop codon mutation and immediately following, and whether the cell line responded to read-through treatment.
| Cell line name | Mutation | Condition | Stop codon | Nucleotide after PTC | Read-through |
|---|---|---|---|---|---|
| RDEB53K | p.R669X/p.R669X | HpV immortalized, cSCC | C->T TGA |
G | NO |
| RDEB14K | p.Q251X/p.Q251X | HpV immortalized | C->T TAG |
T | YES |
| RDEB13K | p.G2073D/p.R578X | HpV immortalized | C->T TGA |
G | YES |
| RDEB101K/F | p.R669X/p.R669X | Primary, HpV immortalized | C->T TGA |
G | NO |
| RDEB102K/F | p.R2814X/p.R2814X | Primary, HpV immortalized | C->T TGA |
C | YES |
| RDEB5K | p.R578X/p.Q906X | Primary | C->T TGA/TAG |
G/G | YES |
| RDEB3K | p.R525X/- | cSCC | C-> T TGA |
G | NO |
| RDEB107F | p.R578X/p.R2492X | HpV immortalized | C->T TGA/TGA |
G/G | NO |
| RDEB111K | p.R2610X/p.R2610X | HpV immortalized | C->T TGA |
G | YES |
| RDEB112K | p.R1343X/p.R2069C | HpV immortalized | C->T TGA |
G | YES |
| RDEB110K | p.R2338X/p.R2338X | HpV immortalized | C->T TGA/TGA |
G | NO |
| RDEB113K | p.R1630X/p.R2069C | HpV immortalized | C->T TGA |
G | YES |
| RDEB115K | c.4249delG/p.R1933X | HpV immortalized | C->T TGA |
G | YES |
| RDEB116K | p.R1933X/p.R1933X | HpV immortalized | C->T TGA |
G | NO |
Figure 1.
Amlexanox induces full length type VII collagen synthesis in RDEB patient derived cells. Keratinocytes (a and b) and fibroblasts (c) isolated from different RDEB patients were treated with 250 μM amlexanox (A) or gentamicin 1 mM (G). Expression of type VII collagen after treatment was compared to normal human keratinocytes or fibroblasts (NHK or NHF) using Western blotting. For optimization of detection, the amount of protein loaded from RDEB keratinocytes (not fibroblasts) was twice the amount of protein loaded from control cells. Levels of type VII collagen expression were quantified in image J relative to β-actin and non-treated controls (NT). Graph shows protein levels as a percentage of normal cells.
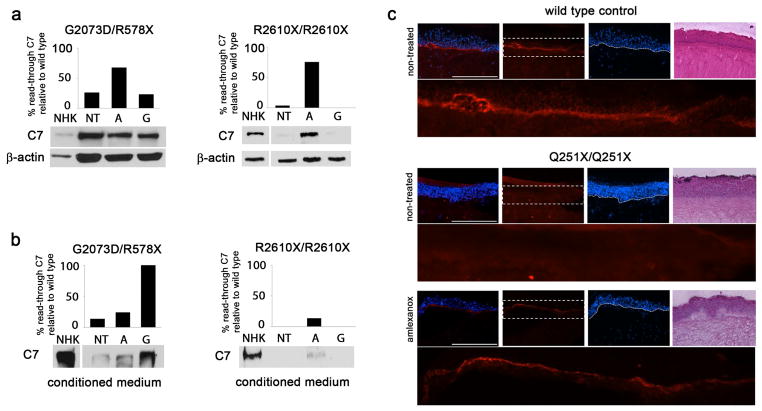
Figure 2.
Read-through type VII collagen is stable and locates to the dermal-epidermal junction. RDEB cells harboring G2073D/R578X mutations and R2610X/R2610X mutations were assessed for levels of intracellular type VII collagen (a) and levels of secreted type VII collagen (b) using Western blotting. Treatment with amlexanox and gentamicin stabilizes secreted levels of the protein. For optimization of detection, the amount of protein loaded from RDEB cells was twice the amount of protein loaded from control cells. (c) Treatment of organotypic skin cultures with amlexanox for two weeks increases type VII collagen deposition at the dermal-epidermal junction (DEJ). Organotypic skin cultures were prepared using RDEB cells or wild type cells, and synthesis of type VII collagen at the DEJ was evaluated using immunofluorescence staining. The magnification bar = 400 μM.
Read-through type VII collagen is stable in cell culture media and localizes to the dermal-epidermal junction in organotypic culture
To evaluate whether read-through type VII collagen protein is functional, Western blotting of proteins isolated from cell culture media was performed and fibrinogen based organotypic skin equivalent cultures using either RDEB or normal cells were prepared. Substitution of a pathogenic missense mutation in place of a PTC could affect proper assembly and post-translational modification of type VII collagen. Collagen triple helix formation is necessary to protect type VII collagen monomers from proteolytic degradation after secretion (Christiano et al., 1996) and mutations in the non-collagenous domains can affect protein-protein binding at the BMZ (Brittingham et al., 2006). Western blotting of proteins collected from p.G2073D/p.R578X and p.R2610X/p.R2610X keratinocyte culture media after treatment with amlexanox and gentamicin showed increased levels of full length type VII collagen (Figure 2b), demonstrating stability of read-through protein.
To assess correct dermal-epidermal junction (DEJ) deposition of read-through type VII collagen, organotypic cultures were prepared and treated with amlexanox for 2 weeks. Read-through type VII collagen at the DEJ was then evaluated by immunofluorescence using a type VII collagen specific antibody raised against the N-terminal, non-collagenous domain. Notable increase in type VII collagen at the DEJ in the RDEB p.Q251X/p.Q251X culture was apparent after two weeks of treatment (Figure 2c). An increase in type VII collagen synthesis in RDEB p.R578X/p.Q906X organotypic culture was also observed (Figure S3), however, a significant amount of intracellular type VII collagen was also detected making it difficult to identify a difference at the DEJ. The RDEB culture prepared with G2073D/R578X cells did not show a clear difference in type VII collagen synthesis between treated and non-treated control, which is likely due to the high levels of intracellular protein previously detected by Western blotting (Figure 2a) making it difficult to distinguish an increase at the DEJ (Figure S3).
Amlexanox increases COL7A1 transcript levels and phosphorylation of the RNA helicase UPF1
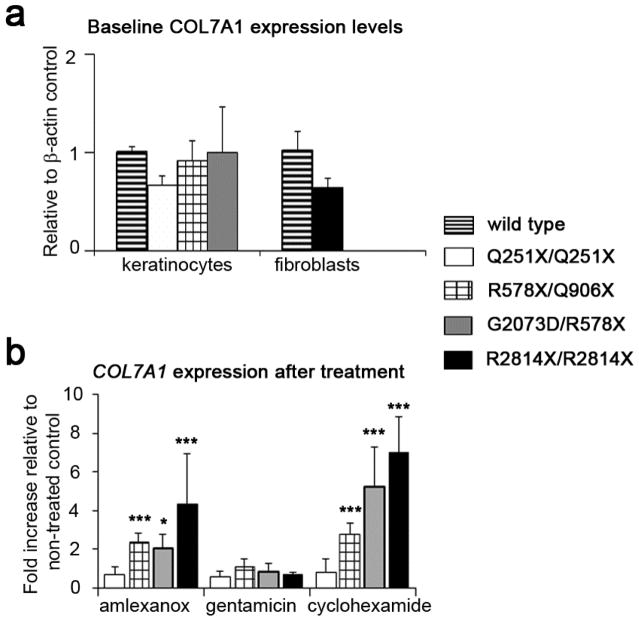
Amlexanox has previously been shown to inhibit nonsense mediated mRNA decay (NMD) in cell based reporter assays (Gonzalez-Hilarion et al., 2012). To investigate whether amlexanox increases COL7A1 transcript levels, potentially through inhibition of NMD, COL7A1 mRNA was measured using quantitative-PCR (QPCR). Overall base-line levels of COL7A1 were not consistently reduced in PTC harboring RDEB keratinocytes while the single RDEB fibroblast line tested had around 80% of wild-type levels (Figure 3a). Treatment with amlexanox, but not gentamicin, significantly increased mRNA abundance in both RDEB keratinocytes and fibroblasts with the exception of p.Q251X/p.Q251X keratinocytes. In those cells (3 out of 4) that did show increase in transcript levels after amlexanox treatment, COL7A1 expression was around 2-fold or higher compared to untreated control cells (Figure 3b). Cycloheximide was used as a positive control for increase in transcript levels as previously described (Schneider-Poetsch et al., 2010).
Figure 3.
Amlexanox increases transcript levels of COL7A1 in 3 out of 4 RDEB patient cells. (a) Evaluation of baseline levels of COL7A1 transcript compared to normal keratinocytes and fibroblasts shows relatively similar levels of expression. (b) Treatment with amlexanox (250 μM) increases COL7A1 transcript in all but one population of RDEB cells. Gentamicin (1 mM).
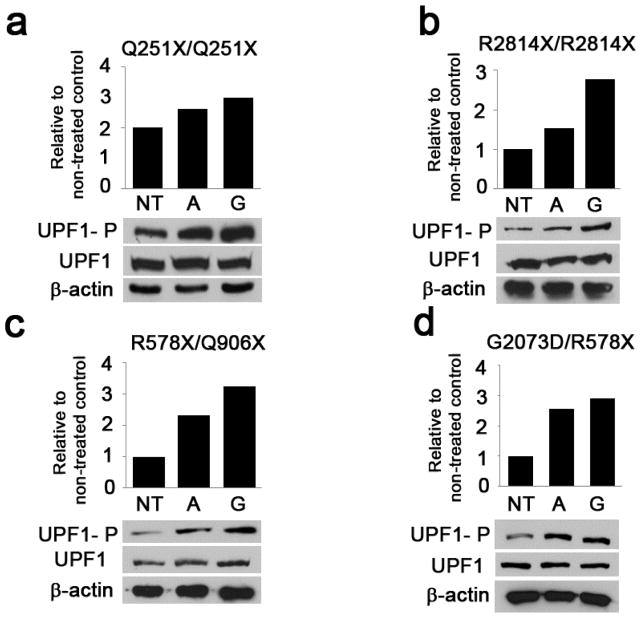
UPF1 is an RNA helicase essential for NMD triggered by premature termination of translation upstream of the last exon-exon junction (Isken et al., 2008). It has been shown that stabilization of either the non-phosphorylated or the phosphorylated form of UPF1 inhibits NMD (Kurosaki et al., 2014). As amlexanox increased transcript levels of COL7A1 in 3 out of 4 RDEB cells, UPF1 levels were evaluated by Western blotting. Read-through efficiency correlated with an increase in phosphorylated form of UPF1 (UPF1-P) in all RDEB cells tested Figure 4. No increase in UPF1-P was observed in RDEB cells which did not result in read-through with amlexanox treatment (data not shown). Increase in UPF1-P was lowest for the single cell population which did read-through but did not exhibit increased transcript levels after amlexanox treatment (p.Q251X/p.Q251X keratinocytes).
Figure 4.
Read-through synthesis of type VII collagen correlates with increase in UPF1 phosphorylation state. RDEB or wild type cells were cultured in 100 mm dishes and treated with either amlexanox or gentamicin for 48 hours. Levels of UPF1-P were quantified in image J relative to total levels of UPF1 and non-treated controls.
DISCUSSION
Previous studies have reported that PTC read-through is both codon and context dependent (Manuvakhova et al., 2000; McCaughan et al., 1995). Not only the specific sequence of the stop codon but also the nucleotide immediately following the stop codon has been shown to affect the amount of read-through product. In addition, endogenous (natural) read-through is a mechanism that governs protein isoform expression, most notably demonstrated in Drosophila (Jungreis et al., 2011), and is both tissue specific and dependent on the 3′ nucleotide context (Prieto-Godino et al., 2016). These findings suggest that the termination of translation is dependent on factors in addition to the precise trinucleotide sequence of the PTC. The data presented here agree with results pertaining to 3′ nucleotide context when compared with other read-through studies (Table 1). Among the stop signals tested so far the highest efficacy of read-through was observed in the UGA-C sequence and lowest, or no efficacy in the UGA-G stop codon combination (Table 1). However, there was inconsistency with p.R578X and p.R1933X mutations which did not read-through in two samples (RDEB107F and RDEB116K) yet did show read-through in three other samples (RDEB13K, RDEB5K, and RDEB115K) even though in each case the stop codon and the 3′ nucleotide were identical. In this respect it is of interest to note that those RDEB cells which showed read-through with amlexanox also showed read-through with gentamicin and had endogenous full length type VII collagen in the untreated control, presumably as result of endogenous “leaky” PTC mutation (albeit at much lower levels). This observation suggests that read-through is primarily possible in those cells with an endogenous level of read-through and that this read-through is dependent on as yet unidentified factors. Such factors may well be potential disease modifiers in RDEB patients with PTC mutations. Additionally, these observations may imply that both gentamicin and amlexanox are acting to enhance read-through of PTC mutations in COL7A1 rather than initiating read-through.
When comparing gentamicin with amlexanox, the data presented here demonstrate that amlexanox treatment results in greater levels of full length type VII collagen in 8/10 cells showing read-through while gentamicin treatment resulted in greater levels in 2/10 cells. We attribute this to different mechanisms of action, as amlexanox has been identified as an inhibitor of NMD (Gonzalez-Hilarion et al., 2012) while gentamicin, as an aminoglycoside, is thought to act on the ribosome (Yoshizawa et al., 1998). Indeed, mRNA data at 24 hours after treatment (Figure 3b) identifies increase with amlexanox and not gentamicin, in keeping with previous studies showing gentamicin induces transcript increase after 48 hours (Baradaran-Heravi et al., 2016), presumably due to feedback as a result of read-through.
Although it has not been demonstrated which amino acid is inserted into the site of the PTC, the increase in type VII collagen harvested from amlexanox treated RDEB culture medium suggests that read-through type VII collagen is both stable and functional (Figure 2b). This conclusion is supported by correct linear deposition of read-through type VII collagen at the DEJ in organotypic culture (Figure 2c). This is not surprising considering that most mutations which affect the stability of the protein result in glycine substitutions in the collagen triple helix (Christiano et al., 1996; Cserhalmi-Friedman et al., 1997) and the mutations tested here did not affect glycine residues (Table 1).
It has been suggested that around 30% of wild type levels of type VII collagen provide normal function in a murine model of RDEB (Nystrom et al., 2013) and that type VII collagen has a half-life of ~30 days (Kuhl et al., 2016). The study presented here shows that the amount of read-through type VII collagen synthesized after a 48 hour treatment with amlexanox in vitro varies between 8% and 80% (Figure 1), as compared to healthy control cells. These results are promising since even a low level of read-through type VII collagen could potentially accumulate with continued treatment, and improve the adhesion of epidermis and dermis at the DEJ over time, resulting in improved quality of life for patients.
It should be pointed out that the concentration of amlexanox used in this study is higher than that described in the serum of animals receiving 100 mg/kg oral amlexanox, which results in >5μM serum concentration (Reilly et al., 2013), and is a lower concentration than 50μM which is the lowest dose examined here, and shown to lead to read-through (Figure S2). Consequently, further work will be required to ascertain the correct, tolerable dose, needed to elicit read-through in RDEB patients.
Other compounds which have shown ability to read-through PTC mutations are in clinical use, most notably PTC124 (ataluren) which was approved for treatment of Duchenne muscular dystrophy (Finkel, 2010). However, two independent studies have shown that PTC124 is not able to induce read-through of PTC mutations in COL7A1, thus suggesting PTC124 will not be suitable for RDEB (Cogan et al., 2014; McElroy et al., 2013).
Recent clinical trials have raised hope for RDEB patient treatment but a cure remains elusive as a result of complications and limitations within each approach (Uitto et al., 2016). Amlexanox is an FDA approved drug for mouth ulcers and currently in phase II clinical trial for type II diabetes and for non-fatty liver disease (www.clinicaltrials.gov) where patients are treated orally (25 mg/kg) with no adverse side effects reported. Because amlexanox has been in clinical use both topically and orally, drug pharmacokinetics and toxicity profiles have already been established. That, coupled with the pre-clinical data reported here, suggests the possibility of rapid translation into clinical practice in RDEB patients. Furthermore, amlexanox has been shown to possess anti-inflammatory properties (Saijo et al., 1985) which may have additional benefit for patients in the current context. Indeed, it is conceivable that a reduction in inflammatory processes may reduce proteolytic degradation within the environment of RDEB wounds leading to improved stability of type VII collagen.
In conclusion, this study demonstrates the feasibility of amlexanox to synthesize full length type VII collagen by read-through of PTC mutations in COL7A1 in RDEB patient derived cells. The data show that amlexanox could potentially benefit RDEB patients by gradually increasing type VII collagen levels at the DEJ while circumventing adverse side effects reported for other read-through approaches.
MATERIALS & METHODS
Cell cultures
Cells were isolated from skin biopsies taken as part of routine surgical or diagnostic procedures. Informed written consent was obtained from each patient or in the case of under-aged children from their parents or guardian. This study was performed in accordance with the Helsinki declaration. All cells were cultured at 37° C at 5% CO2. Human fibroblasts were grown in Dulbecco’s modified essential medium (DMEM, Corning cellgro, Mediatech Inc, Manassas, VA) supplemented with 10% fetal bovine serum (FBS, PEAK Serum, Cat PS-FB1, Colorado, USA). Keratinocytes and fibroblasts were immortalized using the E6 and E7 genes of human papillomavirus as described (Halbert et al., 1991). RDEB and normal keratinocytes were cultured in DMEM/Ham’s F12 medium (3:1) supplemented with 10% FBS, 10 ng/ml of epidermal growth factor, 10−10 M cholera toxin, 0.4 μg/ml of hydrocortisone, 5 μg/ml of transferrin, 5 μg/ml of insulin and 5 μg/ml liothyronine. All media contained ascorbic acid (150 μM).
Drug treatment and western blot analysis
Fibroblasts or keratinocytes were plated in a 100 mm dish at 8×105 to achieve 70–80% confluence the following day, when medium was changed with vehicle or either 250 μM amlexanox (Adipogen Life Sciences, San Diego, CA) or 1 mM gentamicin (G1264, Sigma Aldrich, St. Louis, MO). Medium was then changed daily containing freshly dissolved drug and after 48 hours of treatment cells were lysed with radioimmunoprecipitation assay buffer. Lysate was placed in a centrifuge for 5 minutes at 4°C, and the supernatant was mixed with a 6x Laemmli loading buffer. Before loading onto SDS-PAGE, the samples were boiled for 5 minutes at 95°C. For type VII collagen detection, 90 μg of protein was loaded on a 6% acrylamide gel. Primary antibodies used were: polyclonal rabbit antibody raised against the NC1 domain of type VII collagen (kindly provided by Dr. Mei Chen, USC) (dilution 1:4,000); UPF1 (Cell Signaling, Beverly, MA) (dilution 1:1,000), UPF1-P (Ser1127, EMD Millipore, Billerica, MA) (dilution 1:1,000); and β-actin – (Santa Cruz Biotechnology, Dallas, TX) (dilution 1:5,000). Resolved proteins were transferred onto nitrocellulose membrane with a BioRad Trans-Blot-Turbo (Bio-Rad, Hercules, CA), blocked in PBS-0.1% Tween with 5% milk or 5% BSA according to requirements of the primary antibody, and incubated overnight with the primary antibody. After incubation with IgG-HRP conjugated secondary antibody (Santa Cruz Biotechnology), membrane was incubated with Pierce ECL Western blotting substrate (Fisher Scientific, Waltham, MA) and exposed to CL-XPosure X-ray film (Fisher Scientific, Waltham, MA).
Protein Quantification
Total cell lysates were quantified with Pierce bicinchoninic assay Protein Assay kit (Fisher Scientific, Waltham, MA) and 90 μg of protein were loaded onto SDS-PAGE gel. Western blot signal was quantified with Image J. Type VII collagen was quantified relative to β-actin and to non-treated control. UPF1-P was quantified relative to total UPF1 protein amount and non-treated control.
Metabolic activity assay
Cells were seeded in 96-well plate (1×104 cells/well) and incubated with given doses of the respective drug for 48 hours as described. After 48 hours the medium was changed with DMEM 10% FBS + WST-1 substrate (Roche Diagnostics, Manheim, Germany) (final WST-1 concentration 10%). After 30 minutes incubation at 37°C the absorbance was measured using Flex Station 3 plate reader (Molecular Devices, Sunnyvale, CA) at 450 nm.
Live/Dead Cell Assay
Cells were seeded in a black 96 well plate (3×104 cells/well) and treated over 48 hours with amlexanox. After 48 hours a working solution of 6 μM calcein AM and 6 μM EtD-III was added to the cells according to manufacturer’s instructions (EarlyTox Live/Dead Assay; Molecular Devices, Sunnyvale, CA). After 40 minutes of incubation at room temperature in the dark, fluorescence was measured with Flex Station 3 plate reader (Molecular Devices, Sunnyvale, CA) at two wavelengths – excitation at 495 nm and emission at 530 nm; excitation at 530 nm and emission at 645 nm.
qRT-PCR
Keratinocytes and fibroblasts were plated in six well plates for 90% confluence the next day. Cells were treated with the respective drug for 24 hours and total RNA was isolated using RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. RNA extractions were quantified using a NanoDrop Spectrophotometer (Fisher Scientific, Waltham, MA) and 1.5 μg RNA was used for cDNA synthesis using SuperScript III First-Strand Synthesis System (Invitrogen, Life Technologies, Carlsbad, CA). For detection of COL7A1 expression, the following primers were used: forward primer GGCTGCAATTCTCCATGTGG, and reverse CTGTGAGGCAACTCGCTTCA. For ACTB amplification, the following primers were used: forward CATGTACGTTGCTATCCAGGC, and reverse CTCCTTAATGTCACGCACGAT. For qPCR, SYBR Select Master mix (Life technologies, Carlsbad, CA) was used and cDNA samples were diluted 1:25 to serve as template. Experiments were performed in triplicate.
Organotypic skin equivalents
Bovine fibrinogen (90% clottable, MP Biomedicals, Santa Ana, CA) was dissolved in 1.1% NaCl at 37°C for 4 hours and then filtered with a 0.45 μm nylon membrane filter. Fibroblasts were collected with the use of trypsin and a centrifuge, and re-suspended in media to a final concentration 2×106 cells per ml. 150 μl of this cell suspension was mixed with 1 ml of thrombin (3 IU - Sigma Aldrich, St. Louis, MO), and this cell/thrombin mix was added to fibrinogen at a ratio of 1:1. The mixture was quickly but gently distributed 1 ml/well into a 24-well plate and incubated at 37°C. After 20 minutes medium supplemented with ascorbic acid and aprotinin (Sigma, St. Louis, MO; final concentration 10 μg/ml) was added. The matrices were left to mature for 5–7 days while medium was changed every other day. Keratinocytes were plated on top at 2×106 per well and on the next day the culture was raised to the air-liquid interface on a metal grid and treatment with amlexanox was started; medium was changed every other day with fresh drug, ascorbic acid and aprotinin. Cultures were collected at one or two weeks of treatment and frozen with OCT in liquid nitrogen cooled isopentane. 8 μM sections were cut using a cryostat (AVANTIK QS11) and immunostained with the polyclonal type VII collagen antibody at a dilution of 1:800. Nuclei were counterstained with DAPI (Invitrogen, Carlsbad, CA).
Supplementary Material
Acknowledgments
We thank Leila Youssefian for help with COL7A1 sequencing. We thank the late John Loudon for helpful discussion and scientific insight. This work was supported by DEBRA International and the National Institutes of Health (NIH) - R03 AR067507 and The National Natural Science Foundation of China 81428020.
Abbreviations used
- EB
epidermolysis bullosa
- RDEB
recessive dystrophic epidermolysis bullosa
- NMD
nonsense mediated mRNA decay
- PTC
premature termination codon
- BMZ
basement membrane zone
- DEJ
dermal-epidermal junction
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baradaran-Heravi A, Balgi AD, Zimmerman C, Choi K, Shidmoossavee FS, Tan JS, et al. Novel small molecules potentiate premature termination codon readthrough by aminoglycosides. Nucleic Acids Res. 2016;44:6583–98. doi: 10.1093/nar/gkw638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J, Bornert O, Nystrom A, Gostynski A, Jonkman MF, Aartsma-Rus A, et al. Antisense Oligonucleotide-mediated Exon Skipping as a Systemic Therapeutic Approach for Recessive Dystrophic Epidermolysis Bullosa. Mol Ther Nucleic Acids. 2016;5:e379. doi: 10.1038/mtna.2016.87. [DOI] [PubMed] [Google Scholar]
- Brittingham R, Uitto J, Fertala A. High-affinity binding of the NC1 domain of collagen VII to laminin 5 and collagen IV. Biochem Biophys Res Commun. 2006;343:692–9. doi: 10.1016/j.bbrc.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Bruckner-Tuderman L, Nilssen O, Zimmermann DR, Dours-Zimmermann MT, Kalinke DU, Gedde-Dahl T, Jr, et al. Immunohistochemical and mutation analyses demonstrate that procollagen VII is processed to collagen VII through removal of the NC-2 domain. J Cell Biol. 1995;131:551–9. doi: 10.1083/jcb.131.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Kasahara N, Keene DR, Chan L, Hoeffler WK, Finlay D, et al. Restoration of type VII collagen expression and function in dystrophic epidermolysis bullosa. Nat Genet. 2002;32:670–5. doi: 10.1038/ng1041. [DOI] [PubMed] [Google Scholar]
- Chen M, Marinkovich MP, Jones JC, O’Toole EA, Li YY, Woodley DT. NC1 domain of type VII collagen binds to the beta3 chain of laminin 5 via a unique subdomain within the fibronectin-like repeats. J Invest Dermatol. 1999;112:177–83. doi: 10.1046/j.1523-1747.1999.00491.x. [DOI] [PubMed] [Google Scholar]
- Christiano AM, McGrath JA, Tan KC, Uitto J. Glycine substitutions in the triple-helical region of type VII collagen result in a spectrum of dystrophic epidermolysis bullosa phenotypes and patterns of inheritance. Am J Hum Genet. 1996;58:671–81. [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Uitto J. Type VII collagen: the anchoring fibril protein at fault in dystrophic epidermolysis bullosa. Dermatol Clin. 2010;28:93–105. doi: 10.1016/j.det.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan J, Weinstein J, Wang X, Hou Y, Martin S, South AP, et al. Aminoglycosides restore full-length type VII collagen by overcoming premature termination codons: therapeutic implications for dystrophic epidermolysis bullosa. Mol Ther. 2014;22:1741–52. doi: 10.1038/mt.2014.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserhalmi-Friedman PB, Karpati S, Horvath A, Christiano AM. Identification of the glycine-to-arginine substitution G2043R in type VII collagen in a family with dominant dystrophic epidermolysis bullosa from Hungary. Exp Dermatol. 1997;6:303–7. doi: 10.1111/j.1600-0625.1997.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Fine JD, Bruckner-Tuderman L, Eady RA, Bauer EA, Bauer JW, Has C, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103–26. doi: 10.1016/j.jaad.2014.01.903. [DOI] [PubMed] [Google Scholar]
- Fine JD, Johnson LB, Weiner M, Li KP, Suchindran C. Epidermolysis bullosa and the risk of life-threatening cancers: the National EB Registry experience, 1986–2006. J Am Acad Dermatol. 2009;60:203–11. doi: 10.1016/j.jaad.2008.09.035. [DOI] [PubMed] [Google Scholar]
- Fine JD, Mellerio JE. Extracutaneous manifestations and complications of inherited epidermolysis bullosa: part II. Other organs J Am Acad Dermatol. 2009;61:387–402. doi: 10.1016/j.jaad.2009.03.053. quiz 3–4. [DOI] [PubMed] [Google Scholar]
- Finkel RS. Read-through strategies for suppression of nonsense mutations in Duchenne/Becker muscular dystrophy: aminoglycosides and ataluren (PTC124) J Child Neurol. 2010;25:1158–64. doi: 10.1177/0883073810371129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Hilarion S, Beghyn T, Jia J, Debreuck N, Berte G, Mamchaoui K, et al. Rescue of nonsense mutations by amlexanox in human cells. Orphanet J Rare Dis. 2012;7:58. doi: 10.1186/1750-1172-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M, Sawamura D, Ito K, Abe M, Nishie W, Sakai K, et al. Fibroblasts show more potential as target cells than keratinocytes in COL7A1 gene therapy of dystrophic epidermolysis bullosa. J Invest Dermatol. 2006;126:766–72. doi: 10.1038/sj.jid.5700117. [DOI] [PubMed] [Google Scholar]
- Halbert CL, Demers GW, Galloway DA. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J Virol. 1991;65:473–8. doi: 10.1128/jvi.65.1.473-478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isken O, Kim YK, Hosoda N, Mayeur GL, Hershey JW, Maquat LE. Upf1 phosphorylation triggers translational repression during nonsense-mediated mRNA decay. Cell. 2008;133:314–27. doi: 10.1016/j.cell.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackow J, Titeux M, Portier S, Charbonnier S, Ganier C, Gaucher S, et al. Gene-Corrected Fibroblast Therapy for Recessive Dystrophic Epidermolysis Bullosa using a Self-Inactivating COL7A1 Retroviral Vector. J Invest Dermatol. 2016;136:1346–54. doi: 10.1016/j.jid.2016.02.811. [DOI] [PubMed] [Google Scholar]
- Jungreis I, Lin MF, Spokony R, Chan CS, Negre N, Victorsen A, et al. Evidence of abundant stop codon readthrough in Drosophila and other metazoa. Genome Res. 2011;21:2096–113. doi: 10.1101/gr.119974.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl T, Mezger M, Hausser I, Guey LT, Handgretinger R, Bruckner-Tuderman L, et al. Collagen VII Half-Life at the Dermal-Epidermal Junction Zone: Implications for Mechanisms and Therapy of Genodermatoses. J Invest Dermatol. 2016;136:1116–23. doi: 10.1016/j.jid.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Kurosaki T, Li W, Hoque M, Popp MW, Ermolenko DN, Tian B, et al. A post-translational regulatory switch on UPF1 controls targeted mRNA degradation. Genes Dev. 2014;28:1900–16. doi: 10.1101/gad.245506.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Ivanova L, Zhu H, Yahr A, Ayello J, van de Ven C, et al. Rescue of the mucocutaneous manifestations by human cord blood derived nonhematopoietic stem cells in a mouse model of recessive dystrophic epidermolysis bullosa. Stem Cells. 2015;33:1807–17. doi: 10.1002/stem.1966. [DOI] [PubMed] [Google Scholar]
- Manuvakhova M, Keeling K, Bedwell DM. Aminoglycoside antibiotics mediate context-dependent suppression of termination codons in a mammalian translation system. RNA. 2000;6:1044–55. doi: 10.1017/s1355838200000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaughan KK, Brown CM, Dalphin ME, Berry MJ, Tate WP. Translational termination efficiency in mammals is influenced by the base following the stop codon. Proc Natl Acad Sci U S A. 1995;92:5431–5. doi: 10.1073/pnas.92.12.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy SP, Nomura T, Torrie LS, Warbrick E, Gartner U, Wood G, et al. A lack of premature termination codon read-through efficacy of PTC124 (Ataluren) in a diverse array of reporter assays. PLoS Biol. 2013;11:e1001593. doi: 10.1371/journal.pbio.1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JA, Ishida-Yamamoto A, O’Grady A, Leigh IM, Eady RA. Structural variations in anchoring fibrils in dystrophic epidermolysis bullosa: correlation with type VII collagen expression. J Invest Dermatol. 1993;100:366–72. doi: 10.1111/1523-1747.ep12471830. [DOI] [PubMed] [Google Scholar]
- Mittapalli VR, Madl J, Loffek S, Kiritsi D, Kern JS, Romer W, et al. Injury-Driven Stiffening of the Dermis Expedites Skin Carcinoma Progression. Cancer Res. 2016;76:940–51. doi: 10.1158/0008-5472.CAN-15-1348. [DOI] [PubMed] [Google Scholar]
- Ng YZ, Pourreyron C, Salas-Alanis JC, Dayal JH, Cepeda-Valdes R, Yan W, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522–34. doi: 10.1158/0008-5472.CAN-11-2996. [DOI] [PubMed] [Google Scholar]
- Nystrom A, Velati D, Mittapalli VR, Fritsch A, Kern JS, Bruckner-Tuderman L. Collagen VII plays a dual role in wound healing. J Clin Invest. 2013;123:3498–509. doi: 10.1172/JCI68127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto-Godino LL, Rytz R, Bargeton B, Abuin L, Arguello JR, Peraro MD, et al. Olfactory receptor pseudo-pseudogenes. Nature. 2016;539:93–7. doi: 10.1038/nature19824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly SM, Chiang SH, Decker SJ, Chang L, Uhm M, Larsen MJ, et al. An inhibitor of the protein kinases TBK1 and IKK-varepsilon improves obesity-related metabolic dysfunctions in mice. Nat Med. 2013;19:313–21. doi: 10.1038/nm.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo T, Kuriki H, Ashida Y, Makino H, Maki Y. Mechanism of the action of amoxanox (AA-673), an orally active antiallergic agent. Int Arch Allergy Appl Immunol. 1985;78:43–50. doi: 10.1159/000233861. [DOI] [PubMed] [Google Scholar]
- Schneider-Poetsch T, Ju J, Eyler DE, Dang Y, Bhat S, Merrick WC, et al. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nat Chem Biol. 2010;6:209–17. doi: 10.1038/nchembio.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siprashvili Z, Nguyen NT, Bezchinsky MY, Marinkovich MP, Lane AT, Khavari PA. Long-term type VII collagen restoration to human epidermolysis bullosa skin tissue. Hum Gene Ther. 2010;21:1299–310. doi: 10.1089/hum.2010.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siprashvili Z, Nguyen NT, Gorell ES, Loutit K, Khuu P, Furukawa LK, et al. Safety and Wound Outcomes Following Genetically Corrected Autologous Epidermal Grafts in Patients With Recessive Dystrophic Epidermolysis Bullosa. JAMA. 2016;316:1808–17. doi: 10.1001/jama.2016.15588. [DOI] [PubMed] [Google Scholar]
- Tamai K, Yamazaki T, Chino T, Ishii M, Otsuru S, Kikuchi Y, et al. PDGFRalpha-positive cells in bone marrow are mobilized by high mobility group box 1 (HMGB1) to regenerate injured epithelia. Proc Natl Acad Sci U S A. 2011;108:6609–14. doi: 10.1073/pnas.1016753108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titeux M, Pendaries V, Zanta-Boussif MA, Decha A, Pironon N, Tonasso L, et al. SIN retroviral vectors expressing COL7A1 under human promoters for ex vivo gene therapy of recessive dystrophic epidermolysis bullosa. Mol Ther. 2010;18:1509–18. doi: 10.1038/mt.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokgoz B, Somdas MA, Ucar C, Kocyigit I, Unal A, Sipahioglu MH, et al. Correlation between hearing loss and peritonitis frequency and administration of ototoxic intraperitoneal antibiotics in patients with CAPD. Ren Fail. 2010;32:179–84. doi: 10.3109/08860220903491224. [DOI] [PubMed] [Google Scholar]
- Tolar J, Ishida-Yamamoto A, Riddle M, McElmurry RT, Osborn M, Xia L, et al. Amelioration of epidermolysis bullosa by transfer of wild-type bone marrow cells. Blood. 2009;113:1167–74. doi: 10.1182/blood-2008-06-161299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turczynski S, Titeux M, Tonasso L, Decha A, Ishida-Yamamoto A, Hovnanian A. Targeted Exon Skipping Restores Type VII Collagen Expression and Anchoring Fibril Formation in an In Vivo RDEB Model. J Invest Dermatol. 2016;136:2387–95. doi: 10.1016/j.jid.2016.07.029. [DOI] [PubMed] [Google Scholar]
- Uitto J, Bruckner-Tuderman L, Christiano AM, McGrath JA, Has C, South AP, et al. Progress toward Treatment and Cure of Epidermolysis Bullosa: Summary of the DEBRA International Research Symposium EB2015. J Invest Dermatol. 2016;136:352–8. doi: 10.1016/j.jid.2015.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Akker PC, Jonkman MF, Rengaw T, Bruckner-Tuderman L, Has C, Bauer JW, et al. The international dystrophic epidermolysis bullosa patient registry: an online database of dystrophic epidermolysis bullosa patients and their COL7A1 mutations. Hum Mutat. 2011;32:1100–7. doi: 10.1002/humu.21551. [DOI] [PubMed] [Google Scholar]
- Villone D, Fritsch A, Koch M, Bruckner-Tuderman L, Hansen U, Bruckner P. Supramolecular interactions in the dermo-epidermal junction zone: anchoring fibril-collagen VII tightly binds to banded collagen fibrils. J Biol Chem. 2008;283:24506–13. doi: 10.1074/jbc.M802415200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JE, Ishida-Yamamoto A, McGrath JA, Hordinsky M, Keene DR, Woodley DT, et al. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. N Engl J Med. 2010;363:629–39. doi: 10.1056/NEJMoa0910501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker PD, Shah SV. Gentamicin enhanced production of hydrogen peroxide by renal cortical mitochondria. Am J Physiol. 1987;253:C495–9. doi: 10.1152/ajpcell.1987.253.4.C495. [DOI] [PubMed] [Google Scholar]
- Wertheim-Tysarowska K, Sobczynska-Tomaszewska A, Kowalewski C, Skronski M, Swieckowski G, Kutkowska-Kazmierczak A, et al. The COL7A1 mutation database. Hum Mutat. 2012;33:327–31. doi: 10.1002/humu.21651. [DOI] [PubMed] [Google Scholar]
- Woodley DT, Wang X, Amir M, Hwang B, Remington J, Hou Y, et al. Intravenously injected recombinant human type VII collagen homes to skin wounds and restores skin integrity of dystrophic epidermolysis bullosa. J Invest Dermatol. 2013;133:1910–3. doi: 10.1038/jid.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa S, Fourmy D, Puglisi JD. Structural origins of gentamicin antibiotic action. EMBO J. 1998;17(22):6437–48. doi: 10.1093/emboj/17.22.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.