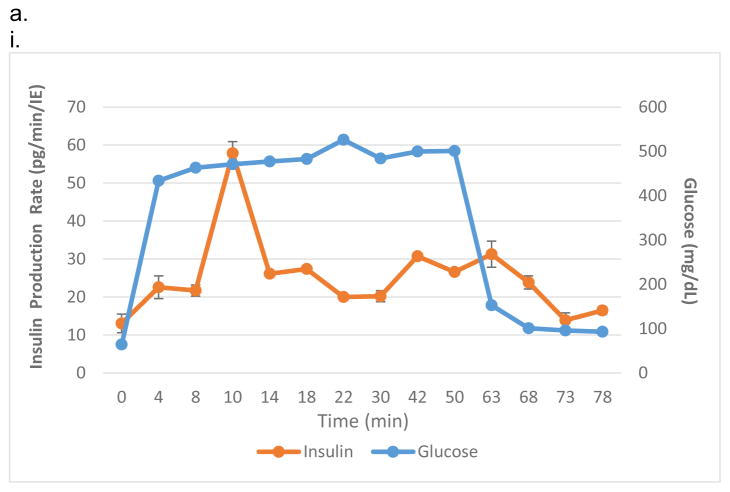
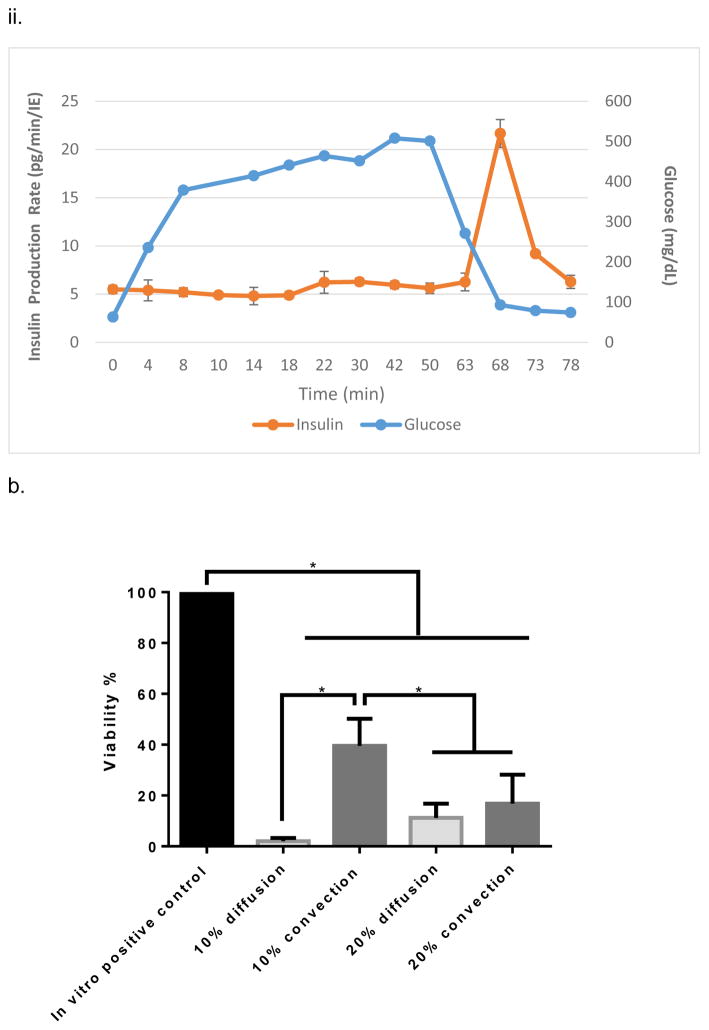
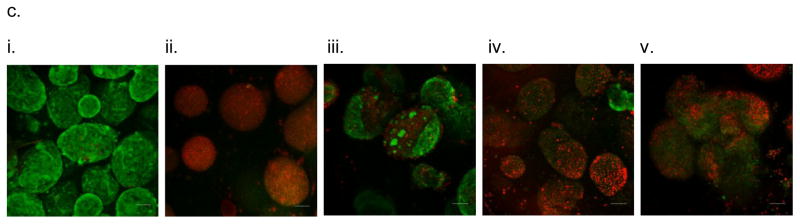
Figure 2.
In vitro testing of the intravascular bioartificial pancreas device (iBAP) with 10% or 20% islet density encapsulated with 10 nm-pore size SNM. (a) Glucose-insulin kinetics of the SNM-encapsulated iBAP with 10% (i) or 20% (ii) islet densities under convection was measured from exposing them to a series of low, high, and low glucose conditions. (b) The SNM-encapsulated iBAP with 10% islet density under convection (10% convection) showed significantly higher viability compared to that of 10% islet density under diffusion (10% diffusion), and 20% islet density under both diffusion (20% diffusion) and convection (20% convection) after 3 days. (n > 3, *p < 0.05). Viabilities of islets that were immediately encapsulated in agarose and dispensed into the islet chamber (IC) without further testing were evaluated as the in vitro positive control. (c) Viable (green) and dead (red) cells were stained for in vitro positive control (i), 10% islet density under diffusion (ii), 10% islet density under convection (iii), 20% islet density under diffusion (iv), and 20% islet density under convection (v) (scale bar = 50 μm). The SNM-encapsulated iBAP with 10% islet density under convection (iii) showed higher viability than that of 10% islet density under diffusion (ii), and 20% islet density under both diffusion (iv) and convection (v). The 10% islet density under diffusion (ii), and 20% islet density under both diffusion (iv) and convection (v) showed similar viability with significant amount of cell death.