Abstract
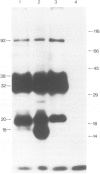
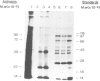
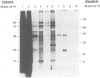
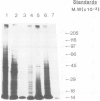
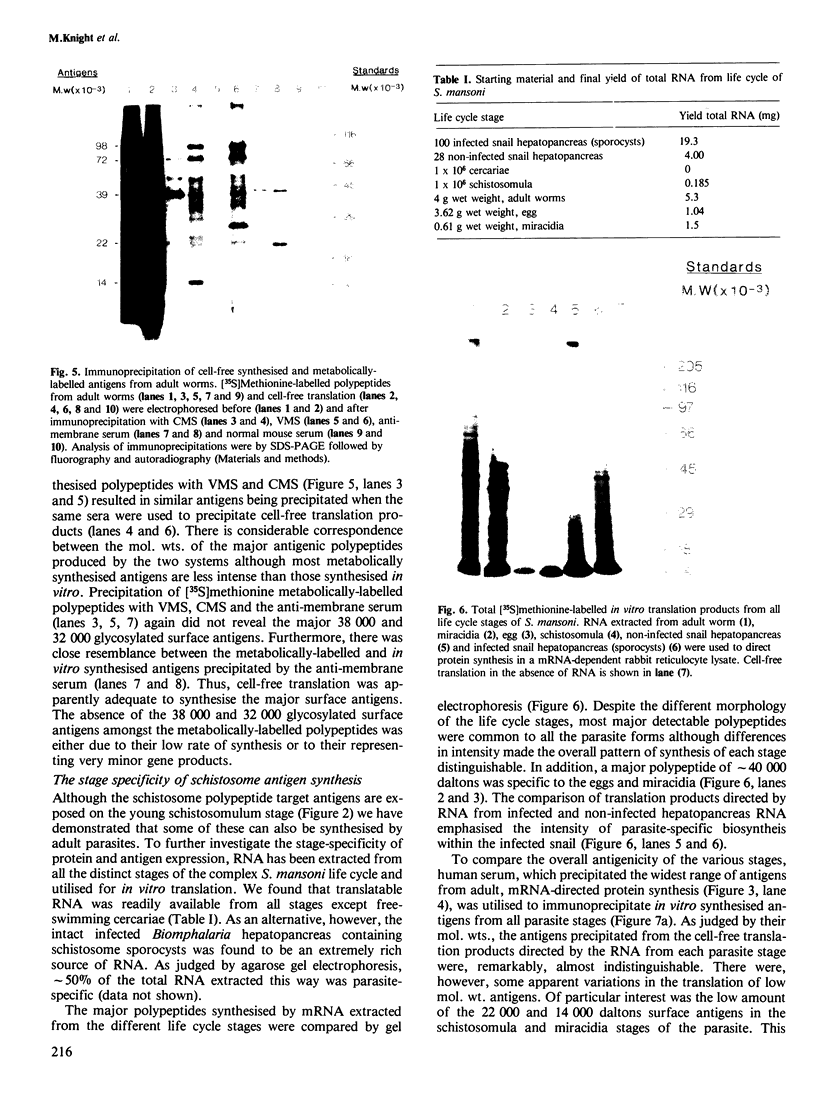
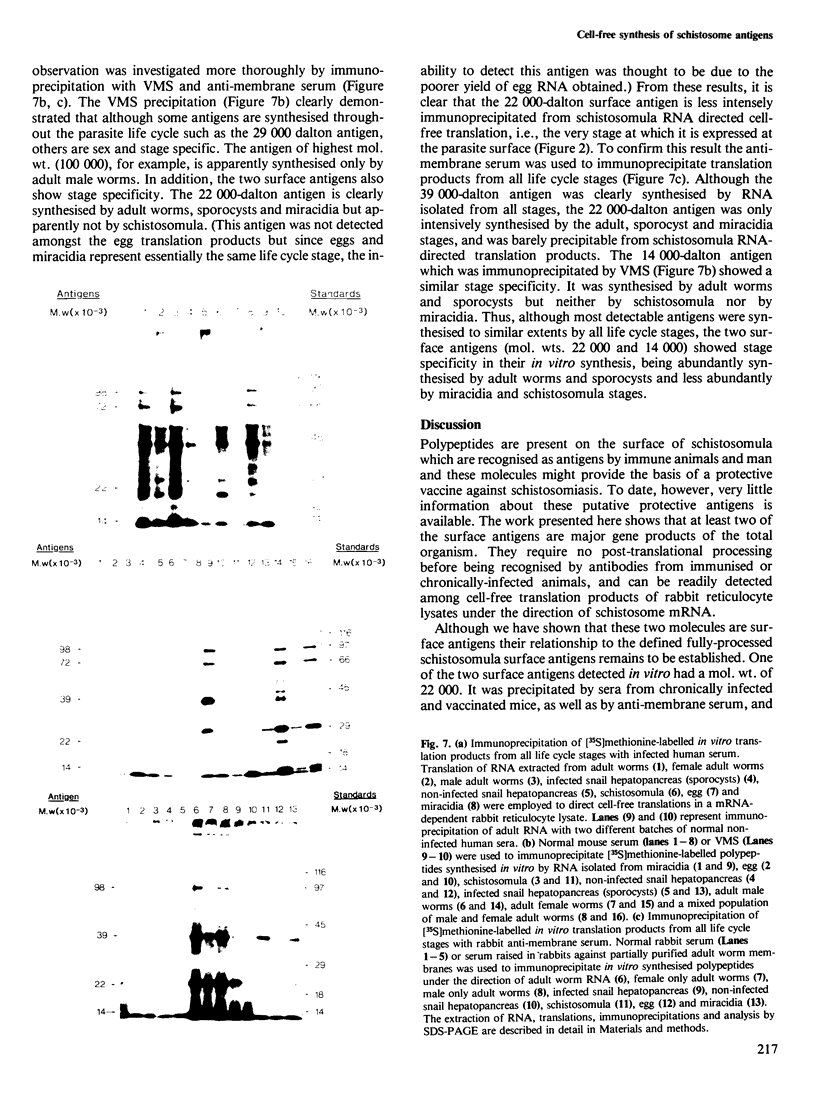
Messenger RNA has been extracted from all stages of the life cycle of the parasitic multicellular helminth Schistosoma mansoni. In vitro translation of these mRNA preparations in rabbit reticulocyte lysates yielded in each case a large number of polypeptides. Immunoprecipitation of translation products either by serum from immune mice or from human patients demonstrated that relatively few, approximately 10, polypeptides are recognised as antigens. Two of the in vitro synthesised antigens, of mol. wts. 22 000 and 14 000, were demonstrated to correspond to schistosomula surface antigens. The expression of these antigens may show stage specificity. Both are readily detected from adult and sporocyst translation products, neither from schistosomula and only the 22 000 antigen from miracidia. This is an unexpected finding since similar polypeptide antigens occur on the surface of schistosomula. These results indicate that not only are schistosomula surface antigens preformed at the preceding sporocyst stage, i.e., within the snail host, but they also remain invariant throughout the life cycle in the vertebrate host. Two other prominent schistosomula surface antigens of mol. wts. 38 000 and 32 000, were not recognised amongst cell-free translation products directed by RNA from any life cycle stage. The demonstration that at least two schistosomula surface antigens are detectable amongst adult mRNA cell-free translation products demonstrates the feasibility of identifying the genes encoding them in cDNA libraries from adult worm mRNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Dissous C., Dissous C., Capron A. Isolation and characterization of surface antigens from Schistosoma mansoni schistosomula. Mol Biochem Parasitol. 1981 Aug;3(4):215–225. doi: 10.1016/0166-6851(81)90053-0. [DOI] [PubMed] [Google Scholar]
- Dissous C., Grzych J. M., Capron A. Schistosoma mansoni surface antigen defined by a rat monoclonal IgG2a. J Immunol. 1982 Nov;129(5):2232–2234. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Grzych J. M., Capron M., Bazin H., Capron A. In vitro and in vivo effector function of rat IgG2a monoclonal anti-S. mansoni antibodies. J Immunol. 1982 Dec;129(6):2739–2743. [PubMed] [Google Scholar]
- Hsü S. Y., Hsü H. F., Osborn J. W. Immunization of rhesus monkeys against schistosome infection by cercariae exposed to high doses of x-radiation. Proc Soc Exp Biol Med. 1969 Sep;131(4):1146–1149. [PubMed] [Google Scholar]
- Majid A. A., Bushara H. O., Saad A. M., Hussein M. F., Taylor M. G., Dargie J. D., Marshall T. F., Nelson G. S. Observations on cattle schistosomiasis in the Sudan, a study in comparative medicine. III. Field testing of an irradiated Schistosoma bovis vaccine. Am J Trop Med Hyg. 1980 May;29(3):452–455. doi: 10.4269/ajtmh.1980.29.452. [DOI] [PubMed] [Google Scholar]
- Minard P., Dean D. A., Jacobson R. H., Vannier W. E., Murrell K. D. Immunization of mice with cobalt-60 irradiated Schistosoma mansoni cercariae. Am J Trop Med Hyg. 1978 Jan;27(1 Pt 1):76–86. doi: 10.4269/ajtmh.1978.27.76. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ramalho-Pinto F. J., Gazzinelli G., Howells R. E., Mota-Santos T. A., Figueiredo E. A., Pellegrino J. Schistosoma mansoni: defined system for stepwise transformation of cercaria to schistosomule in vitro. Exp Parasitol. 1974 Dec;36(3):360–372. doi: 10.1016/0014-4894(74)90076-9. [DOI] [PubMed] [Google Scholar]
- SMITHERS S. R. The isolation of viable schistosome eggs by a digestion technique. Trans R Soc Trop Med Hyg. 1960 Jan;54:68–70. doi: 10.1016/0035-9203(60)90214-5. [DOI] [PubMed] [Google Scholar]
- Simpson A. J., James S. L., Sher A. Identification of surface antigens of schistosomula of Schistosoma mansoni recognized by antibodies from mice immunized by chronic infection and by exposure to highly irradiated cercariae. Infect Immun. 1983 Aug;41(2):591–597. doi: 10.1128/iai.41.2.591-597.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson A. J., Schryer M. D., Cesari I. M., Evans W. H., Smithers S. R. Isolation and partial characterization of the tegumental outer membrane of adult Schistosoma mansoni. Parasitology. 1981 Aug;83(Pt 1):163–177. doi: 10.1017/s0031182000050137. [DOI] [PubMed] [Google Scholar]
- Smithers S. R., Terry R. J. The immunology of schistosomiasis. Adv Parasitol. 1969;7:41–93. doi: 10.1016/s0065-308x(08)60434-0. [DOI] [PubMed] [Google Scholar]
- Taylor D. W., Hayunga E. G., Vannier W. E. Surface antigens of Schistosoma mansoni. Mol Biochem Parasitol. 1981 Jul;3(3):157–168. doi: 10.1016/0166-6851(81)90046-3. [DOI] [PubMed] [Google Scholar]
- Tenniswood M. P., Simpson A. J. The extraction, characterization and in vitro translation of RNA from adult Schistosoma mansoni. Parasitology. 1982 Apr;84(Pt 2):253–261. doi: 10.1017/s0031182000044814. [DOI] [PubMed] [Google Scholar]