Figure 1. The Three Arms of the UPR.
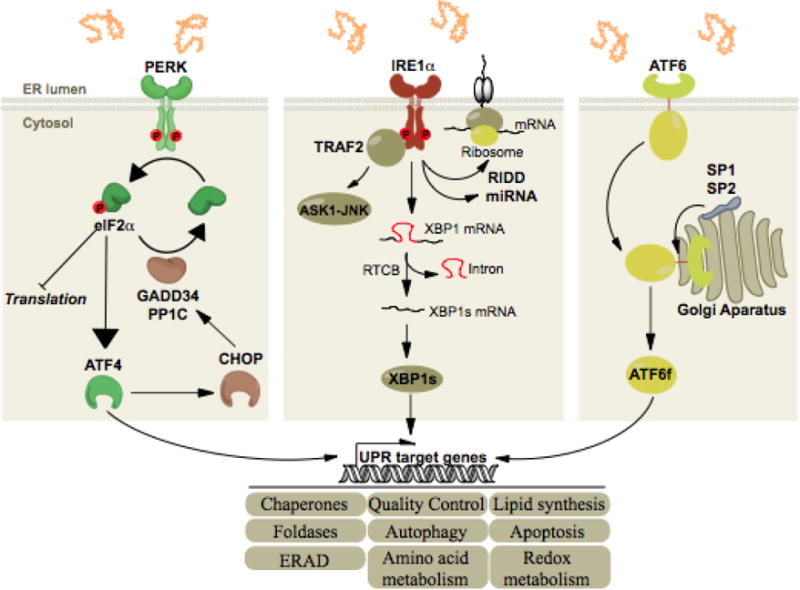
All three ER stress sensors (PERK, IRE1α, ATF6) initially activate signaling events that increase protein-folding capacity and reduce protein load on the ER. These transcriptional and translational outputs tend to re-establish protein-folding homeostasis in the ER and promote cell survival. PERK phosphorylates eIF2alpha, which in turn shut down global translation and in the mean time increases the expression of the transcription factor ATF4. The latter induces the transcription of select genes whose functions are to restore proteostasis (grey boxes) and of CHOP, itself inducing the transcription of GADD34, a regulatory subunit of PP1C. This creates a feedback mechanism leading to the dephosphorylation of eIF2alpha and translation is reinitiated. IRE1α signals through i) the recruitment of TRAF2 leading the activation of the ASK1-JNK cascade and ii) through its RNase via the splicing of XBP1 mRNA or the degradation of RNAs, thereby regulating gene expression at transcriptional and post-transcriptional levels. Finally, upon ER stress, ATF6 is exported from the ER to the Golgi complex where it is cleaved by the proteases S1P and S2P, releasing its cytosolic domain which is a potent transcription factor.
