Fig. 4. Selective recognition of d-sucrose and structures of disaccharides and artificial sugars.
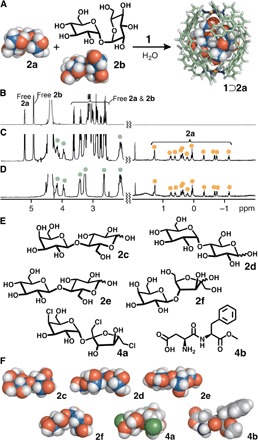
(A) Schematic representation of the selective encapsulation of d-sucrose (2a) from a mixture of 2a and d-trehalose (2b) by capsule 1. 1H NMR spectra (500 MHz, D2O, room temperature) of (B) 2a and 2b, (C) a mixture of 1⊃2a, 2a, and 2b, and (D) isolated 1⊃2a. (E) d-Lactose (2c), d-maltose (2d), d-cellobiose (2e), d-lactulose (2f), sucralose (4a), and aspartame (4b) used as guest molecules and (F) their optimized structures [density functional theory (DFT), B3LYP/6-31G(d); conductor-like polarizable continuum model (CPCM; H2O) level].
