Abstract
Background
Depression is a common complication after stroke, and inflammation may be a pathophysiological mechanism. This study examines whether anti-inflammatory treatment with acetylsalicylic acid (ASA), nonsteroid anti-inflammatory drugs (NSAIDs) or statins influence the risk of depression after stroke.
Methods
A register-based cohort including all patients admitted to hospital with a first-time stroke from Jan. 1, 2001, through Dec. 31, 2011, and a nonstroke population with a similar age and sex distribution was followed for depression until Dec. 31, 2014. Depression was defined as having a hospital contact with depression or having filled prescriptions for anti-depressant medication. The associations between redeemed prescriptions of ASA, NSAIDs or statins with early- (≤ 1 year after stroke or study entry) and late-onset (> 1 year after stroke or study entry) depression were analyzed using Cox proportional hazard regression.
Results
We identified 147 487 patients with first-time stroke and 160 235 individuals without stroke for inclusion in our study. Redeemed prescriptions of ASA, NSAIDs or statins after stroke decreased the risk for early-onset depression, especially in patients with ischemic or severe stroke. Patients who received a combination of anti-inflammatory treatments had the lowest risk for early-onset depression. On the other hand, use of ASA or NSAIDs 1 year after stroke increased the risk for late-onset depression, whereas statin use was associated with a tendency toward a decreased risk.
Limitations
The study used prescription of antidepressant medication as a proxy measure for depression and did not include anti-inflammatory drugs bought over the counter.
Conclusion
Anti-inflammatory treatment is associated with a lower risk for depression shortly after stroke but a higher risk for late depression. This suggests that inflammation contributes differently to the development of depression after stroke depending on the time of onset.
Introduction
Every year more than 10 million people worldwide experience a stroke,1 and depression is a common complication with an 8 times higher incidence than in the general population in the first months after the acute event.2 Comorbid depression seems to have a negative impact on stroke patients’ recovery, mortality and quality of life,2–5 but the link between depression and stroke is still largely unknown. It is possible that brain inflammation contributes to the development of depressive symptoms following stroke.6,7 During the first hours following stroke, proinflammatory cytokines are released in the brain,7–9 and they peak after 3–4 weeks.10 These cytokines may activate the hypothalamus–pituitary–adrenal axis, resulting in increased adrenocorticotropic hormone and cortisol levels11 and elevated conversion of tryptophan into kynurenine instead of serotonin. Hereby the synthesis and availability of serotonin in the brain will be reduced, and this may induce depressive symptoms.6 The depression immediately following stroke may thus be pathogenically different from depression occurring later after stroke.
Owing to the inflammatory component in the pathogenesis, it has recently been suggested that anti-inflammatory medications, such as acetylsalicylic acid (ASA), nonsteroid anti-inflammatory drugs (NSAIDs) and statins, may decrease the risk for depression.12,13 Both ASA and statins are used routinely after ischemic stroke, but to date, only 2 studies have examined the association between statin use and risk for depression after stroke and have reported conflicting findings.14,15
In the present study, we examined the hypothesis that anti-inflammatory treatment with ASA, NSAIDs and statins prescribed during first-time stroke would be associated with decreased risk for subsequent depression. Based on the above-mentioned inflammatory mechanisms, we hypothesized that anti-inflammatory medication would mainly influence depression developing immediately after stroke. Also, as studies have shown that both poststroke levels of C-reactive protein, an acute phase protein, and depression are greater particularly in patients with ischemic stroke,16 we further hypothesized than any influence of anti-inflammatory treatment on depression would be greatest in patients with ischemic stroke or in patients with the most severe strokes. Consequently, we explored associations between anti-inflammatories and time of depression onset, stroke subtypes and disease severity in patients with stroke. The association between anti-inflammatories and time of depression onset was also explored in a nonstroke population with a similar age and sex distribution.
Methods
Study population
We identified all patients in Denmark admitted to hospital with a first-time stroke diagnosis between Jan. 1, 2001, and Dec. 31, 2011, from the Danish National Patient Register using the following International Classification of Diseases (ICD)-10 codes: I61 intracerebral hemorrhage, I63 cerebral infarction, I64 unspecified stroke, and G45 transient ischemic attack (TIA). The Danish National Patient Register was established in 1977 and stores data on diagnosis, treatments and dates from all somatic and psychiatric hospital admissions for every emergency, in- and outpatient contact,17 which allowed us to identify incident stroke cases in the register. We established a comparable nonstroke population with similar sex, age and municipality as patients at the time of stroke diagnosis using information from the Danish Civil Registration System. The Danish Data Inspection approved the study. All data were retrieved from administrative registers, thus informed consent was not required of participants.
Anti-inflammatory treatment
All prescriptions of ASA, NSAIDs, or statins from Jan. 1, 1995, to Dec. 31, 2014, were obtained from the Danish Prescription Register using Anatomic Therapeutic Chemical (ATC) Classification codes B01AC06, B01AC56, N02BA01 and N02BA51 for ASA; N01A for NSAIDs; and C10AA for statins. Anti-inflammatory treatment was measured from 5 years preceding stroke or study entry to either 1) the end of the first month after stroke or study entry, or 2) to the end of the first year after stroke.
Assessment of depression
We obtained information on diagnoses of depression and filled prescriptions for antidepressant medication bewteen Jan. 1, 1995, and Dec. 31, 2014, from the National Patient Register, and we confirmed any fatal cases of stroke from the Danish Cause of Death Register using ICD10 codes F31.3–33 and 34.1 and from the Danish Prescription Register using ATC code N06A. Early-onset depression was defined as a hospital contact with depression or filling a prescription for antidepressants 1 month to 1 year after study entry. Late- onset depression was defined as a hospital contact with depression or filling a prescription for antidepressants 1 year or more after study entry. Of the cases of depression diagnosed after study entry, 0.93% were based solely on an ICD-10 code, 15.19% were based on both an ICD-10 code and an ATC code, and 83.88% were based solely on an ATC code.
Covariates
We included information on basic sociodemographic characteristics, somatic and other psychiatric comorbidity, previous episodes of depression and selected types of medication as covariates. Further, data on stroke severity were available for a subgroup of patients. Education was categorized as basic education (grade 7–9), medium education (high school diploma or vocational degree), higher education (postsecondary degree), or unknown based on data from the Integrated Database for Labor Market Research. Cohabitation status based on data from the Danish Civil Registration System was categorized as single or living with a partner. From the National Patient Registry and the Danish Prescription Register, we obtained information on both hospital admission and medication for somatic or psychiatric comorbidity during the 5 years preceding inclusion in the study. We defined our somatic and psychiatric covariates as somatic/psychiatric status at the time of inclusion. Somatic comorbidity included acute coronary syndrome, other cardiovascular diseases, connective tissue disease, inflammatory disease, infectious disease, cancer, obesity, hypertension, chronic obstructive pulmonary disease, diabetes mellitus and migraine, whereas psychiatric co-morbidity included anxiety, alcoholism, organic brain disorders (including dementia), nonaffective disorders, previous depression, use of lithium and poststroke/postentry delirium. Finally, we included information on prescription of warfarin, clopidogrel and β-blockers after study entry until the end of the first month (for early depression) or first year (for late depression) after study entry. The ICD-10 and ATC codes used for definitions are shown in Appendix 1, Table S1, available at jpn.ca. The covariates were chosen based on a previous analysis of covariates associated with inflammatory diseases and depression following stroke.2 The cardiac medication was chosen as it is assumed to reflect ASA and statin use after acute coronary syndrome and lithium owing to its use for bipolar depression. Anxiety (diagnosis of anxiety disorder, obsessive–compulsive disorder, stress reactions and sleep disorders as well as use of anxiolytic drugs and sedatives), diabetes and cancer (diabetes- and cancer-related neuropathic pain) were also included as covariates, as these diseases are associated with depression and often treated with antidepressant medication. Finally, from the Danish Stroke Registry, including 45 765 patients, we obtained information on stroke severity based on the Scandinavian Stroke Scale. The scale ranges from 0 to 58 points and is based on ratings of consciousness; eye movements; motor power in arms, hands and legs; orientation; speech; facial palsy; and gait.18
Statistical analysis
We used Stata software version 13.1 (StataCorp) for statistical analyses. All analyses were performed separately in stroke patients based on stroke subdiagnosis and in the nonstroke population. However, as the estimates differed only slightly across subdiagnosis, we chose to give the results for all strokes in a single category and included 1 analysis showing the results based on stroke subdiagnosis. Missing data (14% for education) were included as a fixed number. Study entry was the date of stroke event or the corresponding date for the nonstroke population. For early-onset depression, individuals were followed from the end of the first month after study entry until depression, death/emigration or the end of follow-up (1 year after stroke or study entry), whichever came first. Similarly, for late-onset depression, individuals were followed from the end of the first year after study entry until depression, death/emigration or the end of follow-up (Dec. 31, 2014), whichever came first. Individuals who died/emigrated, those who had an episode of depression during the first month after study entry were excluded.
We first tested whether having redeemed a prescription for ASA, NSAIDs or statins at the end of the first month after study entry was associated with decreased risk of early-onset depression in both the stroke and the nonstroke population. We used Cox proportional hazards regressions with follow-up time as the underlying time scale. All analyses were adjusted for covariates, which were entered in multiple regression models using the following stepwise approach. First, the basic sociodemographic characteristics (age, sex, education and cohabitation status) were entered. Then we added somatic comorbidity, other psychiatric comorbidity, previous depression and cardiac medication variables. This allowed us to see the influence of the different groups of covariates on the estimates. The proportional hazards assumption was tested graphically by plotting –ln[–ln(survival)] versus ln(follow-up time), and no violations were found. Furthermore, we used Kaplan–Meier curves to plot cumulative new events of both early- and late-onset depression as a function of follow-up time.
Second, we tested if having redeemed a prescription for ASA, NSAIDs, or statins at the end of the first year after study entry was associated with decreased risk for late-onset depression in the stroke and in the nonstroke populations.
Third, we stratified individuals based on any combined treatment with ASA, NSAIDs and statins and examined how combinations of treatments were associated with the risk for early- or late-onset depression in both populations. The reference group was individuals who did not receive any anti-inflammatory treatment.
Fourth, we repeated the analyses of the combined treatment with ASA, NSAIDs and statins, but stratified by stroke subtype in the stroke population.
Fifth, patients for whom data on stroke severity were available from the Danish Stroke Registry were divided into 2 groups based on stroke severity above or below the median (Scandinavian Stroke Scale score 49), and we examined the association between anti-inflammatory treatment and early- and late-onset depression separately in each group. Interaction was tested using a likelihood ratio test by introducing a 2-factor interaction term (medication use × severity) in the model.
In sensitivity analyses we accounted for individuals who died before depression could have developed using Fine Gray competing risk regression by the stcrreg command in Stata. Finally, we repeated the main analyses defining depression only on the basis of ICD-10 codes and not medication use.
Results
We identified 147 487 patients with first-time stroke during the study period in the Danish National Patient Register as well as a comparable nonstroke population of 160 235 individuals in the Danish Civil Registration System.
In total, 118 156 patients with stroke and 152 185 individuals in the nonstroke population were included in the analyses of early onset depression. Individuals who died/emigrated (stroke: 14 788 [10%]; nonstroke: 46 [< 1%]) were excluded from these analyses along with individuals who had an episode of depression during the first month after study entry (stroke: 14 543 [10%]; nonstroke: 8004 [5%]).
In total, 80 681 patients with stroke and 136 733 in the non-stroke population were included in the analyses of late-onset depression. Individuals who died/emigrated (stroke: 29 532 [20%]; nonstroke: 4140 [3%]) were excluded from these analyses along with individuals who had an episode of depression during the first year after study entry (stroke: 37 274 [25%]; nonstroke: 19 362 [12%]).
In the stroke population, the median duration of follow-up was 365 (range 31–365) days for early-onset depression and 5.5 (range 1.0–14.0) years for late-onset depression. During follow-up, early-onset depression developed in 22%, whereas late-onset depression developed in 17%. For the nonstroke population the median duration of follow-up was 365 (range 31–365) days for early-onset depression and 6.2 (range 1.0–14.0) years in the analyses of late onset depression. During follow-up, early-onset depression developed in 8%, and late-onset depression developed in 15%. Table 1 and Appendix 1, Table S2 show the distribution of the covariates in relation to ASA, NSAID, or statin use and depression outcomes in the stroke and nonstroke populations. Use of ASA and statins seemed strongly associated with comorbidity, particularly in the nonstroke population.
Tabe 1.
Distribution of the covariates in association with ASA, NSAID, or statin use and depression outcomes in patients with stroke*
| Covariate | Total no. | Medication use before the end of the first month, % | Outcome, % | Medication use before end of the first year, % | Outcome, % | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| ASA use (n = 50 693) | NSAID use (n = 33 573) | Statin use (n = 39 861) | Early-onset depression (n = 27 923) | Total no. | ASA use (n = 50 687) | NSAID use (n = 40 747) | Statin use (n = 41 307) | Late-onset depression (n = 80 681) | ||
| Basic characteristics† | ||||||||||
| All | 118 156 | 42.9 | 28.4 | 33.7 | 23.6 | 80 681 | 62.8 | 50.5 | 51.2 | 21.3 |
| Age > 70 yr | 61 357 | 45.2 | 31.2 | 29.1 | 25.1 | 38 444 | 68.6 | 56.8 | 45.9 | 22.5 |
| Women | 56 246 | 42.2 | 30.0 | 31.2 | 26.6 | 36 249 | 61.8 | 55.1 | 47.5 | 23.2 |
| Single | 58 818¶ | 42.2 | 30.0 | 29.1 | 24.8 | 38 187†† | 62.4 | 54.6 | 44.9 | 21.7 |
| Only primary school | 47 954** | 44.2 | 28.7 | 35.3 | 25.0 | 32 354‡‡ | 65.3 | 53.5 | 53.6 | 23.4 |
| Somatic comorbidity‡ | ||||||||||
| Acute coronary syndrome | 5269 | 61.2 | 32.2 | 58.4 | 25.9 | 3278 | 85.9 | 57.7 | 75.3 | 23.8 |
| Other cardiovascular disease | 29 089 | 48.6 | 31.9 | 34.1 | 26.4 | 17 574 | 73.6 | 58.9 | 52.5 | 23.4 |
| Connective tissue disease | 6834 | 43.7 | 30.9 | 34.1 | 25.7 | 4255 | 66.8 | 64.9 | 52.2 | 22.2 |
| Inflammatory disease | 53 061 | 44.8 | 31.8 | 33.3 | 27.3 | 33 112 | 65.1 | 59.1 | 50.0 | 24.3 |
| Infective disease | 88 997 | 43.4 | 29.9 | 34.0 | 24.7 | 59 336 | 63.0 | 53.6 | 51.1 | 22.3 |
| Cancer | 7964 | 41.6 | 33.7 | 28.0 | 25.2 | 4454 | 62.7 | 56.4 | 45.0 | 21.6 |
| Obesity | 4414 | 44.2 | 30.6 | 37.2 | 28.8 | 2935 | 64.3 | 61.2 | 55.3 | 29.0 |
| Hypertension | 68 103 | 46.5 | 30.1 | 36.0 | 25.8 | 43 532 | 69.2 | 55.8 | 54.7 | 22.3 |
| Chronic obstructive pulmonary disease | 21 902 | 44.7 | 30.0 | 34.0 | 27.4 | 13 788 | 64.5 | 58.2 | 50.3 | 24.5 |
| Diabetes mellitus | 13 398 | 47.1 | 29.2 | 43.1 | 26.2 | 8476 | 72.4 | 56.7 | 64.6 | 23.6 |
| Migraine | 3771 | 41.2 | 29.7 | 36.1 | 29.6 | 2493 | 57.5 | 59.9 | 50.3 | 26.9 |
| Psychiatric comorbidity‡ | ||||||||||
| Anxiety | 42 542 | 43.7 | 31.6 | 30.8 | 32.7 | 24 546 | 65.0 | 60.5 | 46.6 | 28.9 |
| Alcoholism | 6003 | 39.2 | 29.4 | 27.2 | 32.3 | 3566 | 54.5 | 56.2 | 38.9 | 28.3 |
| Organic brain disorders (including dementia) | 2872 | 48.1 | 39.6 | 19.7 | 35.0 | 1251 | 65.4 | 64.0 | 28.9 | 18.4 |
| Nonaffective disorder | 7629 | 43.4 | 33.9 | 24.4 | 39.1 | 3713 | 62.3 | 62.2 | 33.9 | 30.2 |
| Poststroke delirium* | 252 | 34.9 | 36.1 | 22.2 | 31.4 | 204 | 60.3 | 66.7 | 35.8 | 19.6 |
| Lithium use | 509 | 40.5 | 30.1 | 27.7 | 56.4 | 176 | 61.9 | 55.7 | 39.8 | 40.3 |
| Previous depression | 26 742 | 42.6 | 29.8 | 33.0 | 47.1 | 11 880 | 61.7 | 57.9 | 50.1 | 33.0 |
| Medication use§ | ||||||||||
| Warfarin use* | 5427 | 42.4 | 29.0 | 45.7 | 16.0 | 8809 | 57.5 | 50.3 | 57.1 | 21.6 |
| Clopidogrel use* | 9777 | 33.0 | 25.6 | 69.5 | 18.3 | 11 305 | 52.1 | 48.1 | 79.6 | 17.3 |
| β-blocker use* | 10 599 | 58.7 | 33.8 | 50.3 | 17.7 | 20 574 | 72.1 | 52.1 | 62.8 | 22.0 |
Based on 118 156 patients with stroke at baseline and 80 681 patients after 1 year.
Measured at baseline.
Measured within 5 years preceeding stroke.
Use from inclusion to the end of the first month.
Data missing for 135 individuals.
Data missing for 17 874 individuals.
Data missing for 117 individuals.
Data missing for 10 546 individuals.
Anti-inflammatory treatment and risk for early-onset depression
In the stroke population, individuals who had filled prescriptions for ASA at the end of the first month after stroke had decreased risk of early-onset depression, with a crude hazard ratio (HR) of 0.76 (95% confidence interval [CI] 0.74–0.78) compared with individuals who had not filled prescriptions for ASA (Fig. 1). The corresponding HRs for NSAID and statin use at the end of the first month after stroke were 0.98 (95% CI 0.95–1.00) and 0.71 (95% CI 0.70–0.73), respectively. Among the stroke population, adding covariates to the crude model did not change the risk estimates for ASA, whereas the estimates were attenuated slightly for statins and became stronger for NSAIDs. In the nonstroke population, ASA, NSAID and statin use was associated with an increased risk of early-onset depression. After adjustment for comorbidity, NSAID use remained significantly associated with increased risk for early-onset depression, with a HR of 1.08 (95% CI 1.04–1.13), whereas use of ASA (HR 0.97, 95% CI 0.92–1.01) and statins (HR 1.00, 95% CI 0.94–1.05) was not associated with risk for early-onset depression (Fig. 1). Appendix 1, Figure S1 shows Kaplan–Meyer plots of the time until early-onset depression in relation to ASA, NSAID, or statin use.
Fig. 1.
Risk for early-onset depression (depression between 1 mo and 1 yr after inclusion) after use of acetylsalicylic acid (ASA), nonsteroidal antiinflammatory drugs (NSAIDs), or statins at the end of the first month after stroke/study entry in 118 156 patients with stroke and 152 185 individuals in the nonstroke population. The crude model 1 is unadjusted. Model 2 is model 1 adjusted for basic covariates (age, sex, education, and cohabitation status). Model 3 is Model 2 with further adjustment for somatic comorbidity (acute coronary syndrome, other cardiovascular disease, connective tissue disease, inflammatory disease, infection, cancer, obesity, hypertension, chronic obstructive pulmonary disease, diabetes mellitus, migraine). Model 4 is Model 3 with further adjustment for psychiatric comorbidity (anxiety, alcoholism, dementia, nonaffective disorders, lithium use, or delirium after stroke/study entry). Model 5 is Model 4 with further adjustment for previous depression (depression before stroke/study entry). The final fully adjusted model is Model 5 with further adjustment for medication use (warfarin, clopidogrel and β-blockers after stroke/study entry). CI = confidence interval; HR = hazard ratio.
Anti-inflammatory treatment and risk for late-onset depression
In the stroke population, individuals who had filled prescriptions for either ASA or NSAIDs at the end of the first year after study entry had increased risk for late-onset depression, with crude HRs of 1.28 (95% CI 1.25–1.33) and 1.48 (95% CI 1.44–1.53), respectively, whereas individuals who had filled prescriptions for statins had decreased risk of late-onset depression (HR 0.90, 95% CI 0.87–0.93). Multiple adjustments had only minor influence on these risk estimates (Fig. 2). In the nonstroke population, risk estimates were similar to those in the stroke population, but the adjustment for covariates attenuated the estimates somewhat (ASA: HR 1.12, 95% CI 1.09–1.16; NSAIDs: HR 1.20, 95% CI 1.17–1.23; statins: HR 0.90, 95% CI 0.86–0.94; Fig. 2). Appendix 1, Figure S2 shows Kaplan–Meyer plots of the time until late-onset depression in relation to ASA, NSAID, or statin use.
Fig. 2.
Risk for late-onset depression (depression after 1 year after inclusion) after use of acetylsalicylic acid (ASA), nonsteroidal antiinflammatory drugs (NSAIDs), or statins at the end of the first year after stroke/study entry in 80 681 patients with stroke and 136 733 individuals in the nonstroke population. The crude Model 1 is unadjusted. Model 2 is Model 1 adjusted for basic covariates (age, sex, education, and cohabitation status). Model 3 is Model 2 with further adjustment for somatic comorbidity (acute coronary syndrome, other cardiovascular disease, connective tissue disease, inflammatory disease, infection, cancer, obesity, hypertension, chronic obstructive pulmonary disease, diabetes mellitus, migraine). Model 4 is Model 3 with further adjustment for psychiatric comorbidity (anxiety, alcoholism, dementia, nonaffective disorders, lithium use, or delirium after stroke/study entry). Model 5 is Model 4 with further adjustment for previous depression (depression before stroke/study entry). The final fully adjusted model is Model 5 with further adjustment for medication use (warfarin, clopidogrel, and β-blockers after stroke/study entry). CI = confidence interval; HR = hazard ratio.
Combined anti-inflammatory treatment and risk for early- and late-onset depression
In the stroke population, individuals who had filled prescriptions for either a single medication or a combination of the anti-inflammatory medications at the end of the first month after stroke had decreased risk for early-onset depression compared with individuals who had never filled prescriptions for any of the medications. Individuals who had filled prescriptions for all 3 medications had the lowest risk, with a risk estimate of 0.63 (95% CI 0.60–0.67) after multiple adjustments (Fig. 3). In the nonstroke population, the corresponding risk estimates suggested no effect of medication use, except for the single use of NSAIDs, which was associated with a slightly increased risk for early-onset depression (HR 1.10, 95% CI 1.05–1.15) after multiple adjustments. For late-onset depression, having filled prescriptions for 1 or more of the medications increased risk for late-onset depression in both the stroke and nonstroke populations, with the exception of single statin use, which was not associated with risk for late-onset depression in the stroke population and further seemed to decrease the risk in the nonstroke population (Fig. 3). In general, the risk estimates were slightly attenuated in the nonstroke population compared with the stroke population. All HRs for each of the covariates in the fully adjusted models are shown in Appendix 1, Tables S3–S6.
Fig. 3.
Risk for early- and late-onset depression (depression 1 month to 1 year after stroke/study entry or more than 1 year after stroke/study entry) after the combined use of acetylsalicylic acid (ASA), nonsteroidal antiinflammatory drugs (NSAIDs), or statins at the end of the first month or at the end of the first year after stroke/study entry. All analyses are adjusted for basic covariates (age, sex, education, and cohabitation status), somatic comorbidity (acute coronary syndrome, other cardiovascular disease, connective tissue disease, inflammatory disease, infection, cancer, obesity, hypertension, chronic obstructive pulmonary disease, diabetes mellitus, migraine), psychiatric comorbidity (anxiety, alcoholism, dementia, nonaffective disorders, lithium use, or delirium after stroke/study entry), previous depression (depression before stroke/study entry), and medication use (warfarin, clopidogrel, and β-blockers after stroke/study entry). CI = confidence interval; HR = hazard ratio.
Combined anti-inflammatory treatment and risk for early- and late-onset depression stratified on stroke subtypes
Among the stroke population included, 8% had intracerebral hemorrhage, 39% had ischemic stroke, 47% had an unspecified stroke (either hemorrhage or infarction) and 6% had TIA. In general, all risk estimates indicated a decreased risk for early-onset depression in individuals who had filled prescriptions for either a single or a combination of the anti-inflammatory medications, with the lowest risk estimates for ischemic stroke and the highest risk estimates for TIA (Fig. 4). Risk estimates for hemorrhagic stroke and TIA had wider CIs because of the lower number of individuals in these groups, and risk estimates for TIA were not statistically significant. For late-onset depression, most risk estimates indicated an increased risk for late-onset depression in individuals who had filled prescriptions for either a single or a combination of the anti-inflammatory medications, with the highest risk estimates for hemorrhagic stroke (Fig. 4). An exception was the single use of a statin, for which risk estimates indicated no effect on risk of late-onset depression in all stroke types except for a tendency toward a decreased risk in individuals with TIA.
Fig. 4.
Risk for early- and late-onset depression (depression 1 mo to 1 yr after or more than 1 yr after stroke/study entry) after the combined use of acetylsalicylic acid (ASA), nonsteroidal antiinflammatory drugs (NSAIDs), or statins at the end of the first month (for early-onset depression) or at the end of the first year (for late-onset depression) after stroke/study entry in patients with stroke based on stroke subtype. All analyses are adjusted for basic covariates (age, sex, education, and cohabitation status), somatic comorbidity (acute coronary syndrome, other cardiovascular disease, connective tissue disease, inflammatory disease, infection, cancer, obesity, hypertension, chronic obstructive pulmonary disease, diabetes mellitus, migraine), psychiatric comorbidity (anxiety, alcoholism, dementia, nonaffective disorders, lithium use, or delirium after stroke/study entry), previous depression (depression before stroke/study entry), and medication use (warfarin, clopidogrel, and β-blockers after stroke/study entry). CI = confidence interval; HR = hazard ratio.
Stroke severity, anti-inflammatory treatment and risk for early- and late-onset depression
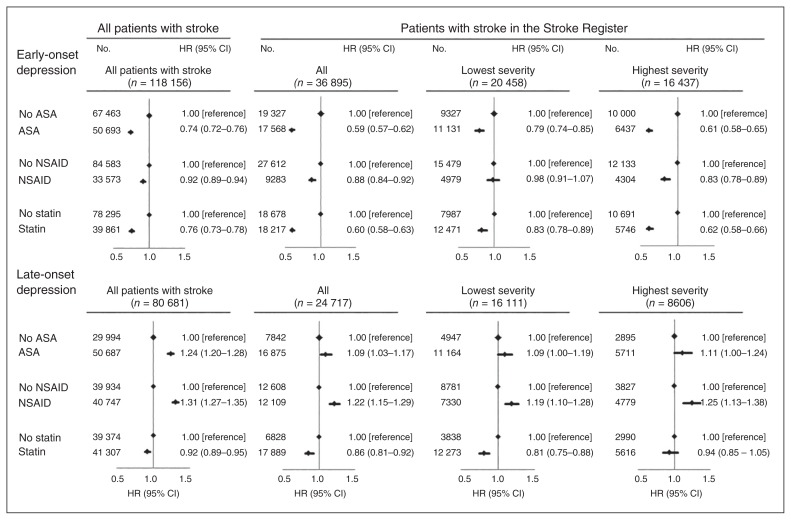
Of the 45 765 patients with stroke in the Danish Stroke Register, 8870 died or experienced an episode of depression before the end of the first month, leaving 36 895 patients with data on stroke severity at risk for early-onset depression. When analyzed together, results from the stroke register were similar to the analyses in all patients combined (Fig. 5). When stratified by stroke severity, risk estimates for early-onset depression were more pronounced in patients with more severe stroke than in patients with less severe stroke in association with ASA, NSAIDs and statins (p < 0.01 for all analyses). When examining risk for late-onset depression, after exclusion of individuals who died or experienced an episode of depression (n = 21 048) before the end of the first year after stroke, 24 717 had data on stroke severity. Overall, results from the stroke register were similar to the results in the entire stroke cohort (Fig. 5). When we stratified for stroke severity, risk estimates were similar when examining prescriptions for ASA and NSAIDs, whereas statins were associated with a more pronounced decreased risk for late-onset depression in patients with less severe stroke (all p < 0.001).
Fig. 5.
Risk for early- and late-onset depression (depression 1 mo to 1 yr after or more than 1 yr after inclusion) after the combined use of acetylsalicylic acid (ASA), nonsteroidal antiinflammatory drugs (NSAIDs), or statins at the end of the first month (for early-onset depression) or at the end of the first year (for late-onset depression) after inclusion in the entire cohort and in patients with stroke with data from the Danish Stroke Register. All analyses are adjusted for basic covariates (age, sex, education, and cohabitation status), somatic comorbidity (acute coronary syndrome, other cardiovascular disease, connective tissue disease, inflammatory disease, infection, cancer, obesity, hypertension, chronic obstructive pulmonary disease, diabetes mellitus, migraine), psychiatric comorbidity (anxiety, alcoholism, dementia, nonaffective disorders, lithium use, or delirium after inclusion), previous depression (depression before inclusion), and medication use (warfarin, clopidogrel, and β-blockers after inclusion). CI = confidence interval; HR = hazard ratio.
Sensitivity analyses
Accounting for competing mortality risk using Fine Gray regression did not change any conclusions (results not shown). Furthermore, when we defined depression only by ICD-10 codes, results were similar, albeit slightly attenuated and with wider confidence intervals (Appendix 1, Tables S7 and S8).
Discussion
This nationwide population-based study showed that having filled a prescription for ASA, NSAIDs, or statins at the end of the first month after stroke decreased the risk of early-onset depression (within the first year), paricularly in patients with more severe stroke. Combined treatment with all 3 types of medication decreased the risk further. Surprisingly, however, ASA or NSAIDs prescribed at the end of the first year after stroke or study entry increased the risk for late-onset depression in the stroke and in the nonstroke populations, whereas statin use seemed to decrease the risk for late-onset depression.
Only 2 studies from 2014 analyzed the risk for depression after stroke, and both assessed statin use after stroke. One cohort study including 423 patients with ischemic stroke found that use of statins shortly after stroke was associated with reduced risk for depression 1 year after stroke.15 In that study, depression was diagnosed based on a validated structured diagnostic interview; however, 32% of the baseline sample was lost to follow-up. The other study including 11 218 Chinese patients with incident stroke and TIA found that regular statin use (60 days within 6 mo of statin use) was associated with increased risk for depression 1 year after stroke.14 However, that study did not account for comorbid diseases and previous depression. To date, no studies have examined the association between use of ASA or NSAIDs and depression after stroke. However, both types of anti-inflammatory treatment have been suggested to decrease the risk for depression in both observational studies and in randomized controlled trials. For ASA, most studies have been observational, and the results have been conflicting.19–23 For NSAIDs, most studies have been randomized controlled trials that have suggested that the NSAID celecoxib decreases the risk for depression.24,25 Among observational studies, a cross-sectional study of 2800 healthy individuals found no association between NSAIDs and depressive symptoms,26 whereas a large Danish study of 123 400 patients taking selective serotonin reuptake inhibitors (SSRIs) found that NSAID use increased the risk for depression.21 These results are similar to those in our nonstroke population, in which NSAID use 1 month after study entry did not influence the risk for early-onset depression, whereas NSAID use 1 year after study entry slightly increased the risk for late-onset depression.
Mechanistically, our results support the hypothesis that increased brain inflammation following the acute attack contributes to the development of depression after stroke.6 This could explain the inconsistent results for early- and late-onset depression in patients with stroke and our findings on stroke severity. The increase in proinflammatory cytokines during the days to weeks following stroke may increase the risk for depression shortly after stroke, possibly through activation of indolamin 2.3-dioxygenase and production of kynurenine instead of serotonin.6 Anti-inflammatory medication, which limits this immediate increase in proinflammatory cytokines, may consequently lower the risk for early depression after stroke. Supporting this finding, patients with TIA and individuals in the nonstroke population did not experience the same benefit from anti-inflammatory medication as patients with stroke, most likely because they did not have the same increase in brain inflammation seen in patients with stroke. Furthermore, risk estimates were more pronounced in patients with ischemic strokes than in patients with hemorrhagic strokes, perhaps because of a higher level of inflammation in ischemic strokes. However, the estimates for hemorrhagic stroke were also less precise. Supporting this finding, a recent meta-analysis suggested that levels of inflammation are higher before ischemic than hemorrhagic strokes.16 In addition, the largest effect of anti-inflammatory medication was seen in patients with more severe stroke, suggesting a larger degree of tissue damage resulting in increased inflammation levels.
Our results on late-onset depression could further support the hypothesis that this type of depression is less driven by inflammation and consequently that anti-inflammatory medication does not protect against this type of depression. In fact, we found an increased risk for late-onset depression in individuals receiving ASA or NSAIDs, but not statins. This finding could have been caused by confounding by indication, as individuals taking anti-inflammatory drugs may have been taking them for a condition that increases the level of inflammation or that is in other ways related to depression. Indeed, this seemed to be more evident in the nonstroke population, where comorbidities were more strongly associated with anti-inflammatory treatment. Furthermore, the individuals who received both ASA and NSAIDs 1 year after stroke were older and had more somatic and psychiatric comorbidities than individuals who did not receive anti-inflammatory medication (data not shown).
Another explanation for our findings could be linked to the mechanism of the medications studied. In general, anti-inflammatory medication has been proposed to exhibit anti-depressant effects by decreasing neuroinflammation.27,28 However, some studies have suggested that inhibition of the cyclooxygenase (COX)-1 and COX-2 by ASA or NSAIDs may increase inflammation in the brain29,30 and augment oxidative and nitro-oxidative stress,27 which in turn may increase the risk for depression. Statins do not act through COX inhibition and thus do not increase the risk for depression through this pathway. This suggests a more complicated association between use of anti-inflammatory agents and risk for depression.
After excluding patients with hemorrhagic stroke (for whom anticoagulant therapy is not recommended), only 67% of the patients with stroke who were still alive 1 year after stroke used ASA, and only 53% used statins even though statin therapy is recommended after ischemic stroke. This is in line with the findings of other studies reporting that not all patients are prescribed medical prophylaxis after stroke.31 The patients who did not receive ASA during the first year after stroke were significantly more often women, were less likely to live alone and were less likely to have cardiovascular disease, diabetes or anxiety than patients who received ASA. Furthermore, they were significantly more likely to use other forms of anticoagulants, such as warfarin and clopidogrel. Patients with stroke who did not receive statins 1 year after stroke were similarly more likely to be women, but the somatic and psychiatric comorbidity was more equal between those receiving and those not receiving statins. Additionally, patients who did not receive statins were less likely to receive other forms of anticoagulants. These findings support the hypothesis that the increased risk for late-onset depression in individuals receiving ASA could be caused by confounding.
Limitations
The strength of this study is the large sample, including all patients in Denmark with first-time stroke between 2001 and 2011 based on information from the National Patient Register, which has high validity for stroke registration.32 Also, we were able to include a nonstroke population and obtain information on comorbid disorders, medication use and stroke severity.
One limitation of the study is that we did not have information on patients in whom depression was diagnosed outside hospitals if they did not fill prescriptions for antidepressants. Such an assumed random misclassification could have diluted our risk estimates. In addition, the study did not include over-the-counter (i.e., non-prescription) medication. Both ASA and NSAIDs can be bought without prescriptions, whereas statins and antidepressant medications can only be bought with a prescription. However, patients with regular use of ASA or NSAIDs would probably buy it on prescription, as they then receive a 50% refund of the cost. In addition, all contacts with the health system, including prescriptions, are free of charge. Thus, the proportion of over-the-counter medication use among our study population was probably small. Another limitation to our study is that prescription of antidepressant medication was used as a proxy for depression, which is not always accurate. For example, SSRIs are often prescribed for other illnesses, such as anxiety and obsessive–compulsive disorder, and tricyclic antidepressants may be prescribed for neuropathic pain. However, all analyses were adjusted for anxiety; obsessive–compulsive disorder; sleep disorders; use of anxiolytics, hypnotics and sedatives; diabetes; and cancer (the latter 2 comorbid illnesses are often associated with neuropathic pain). When we defined depression based only on ICD-10 codes, results were similar. Finally, another limitation was the fact that we had no information on inflammatory levels, smoking, or other risk factors, such as lifestyle factors, in our population, which might have confounded our risk estimates.
Conclusion
Our study provides some evidence that anti-inflammatory treatment after stroke might be associated with a lower risk for early-onset depression, possibly by inhibiting the release of inflammatory cytokines. On the other hand, we found that ASA and NSAID use increased the risk for late-onset depression, which could be explained by confounding by indication or mechanistically by their effect on the COX and augmentation of nitro-oxidative and oxidative stress. Our study suggests that depression within the first year after stroke is driven by increased inflammation in the brain following stroke and stresses the importance of anti-inflammatory treatment with ASA and statins immediate after stroke, as this may not only prevent a new stroke event, but also prevent early-onset depression. Our study also stresses the need for more research on the potential negative effects of anti-inflammatory drugs on the risk for depression.
Footnotes
Funding: The work was supported by the Danish Tryg Foundation [Idn106450] and the Danish Heart Association (Bnr:14-R97-A5003-22834). The sponsors had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Competing interests: None declared.
Contributors: All authors designed the study. M. Osler acquired the data, which all authors analyzed. I. Wium-Andersen and M. Wium-Andersen wrote the article, which all authors reviewed and approved for publication.
References
- 1.Global Burden of Disease Study. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jørgensen TS, Wium-Andersen IK, Wium-Andersen MK, et al. Incidence of depression after stroke, and associated risk factors and mortality outcomes, in a large cohort of Danish patients. JAMA Psychiatry. 2016;73:1032–40. doi: 10.1001/jamapsychiatry.2016.1932. [DOI] [PubMed] [Google Scholar]
- 3.Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psychiatry. 2016;173:221–31. doi: 10.1176/appi.ajp.2015.15030363. [DOI] [PubMed] [Google Scholar]
- 4.Ayerbe L, Ayis S, Wolfe CD, et al. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry. 2013;202:14–21. doi: 10.1192/bjp.bp.111.107664. [DOI] [PubMed] [Google Scholar]
- 5.Shi YZ, Xiang YT, Yang Y, et al. Depression after minor stroke: the association with disability and quality of life–a 1-year follow-up study. Int J Geriatr Psychiatry. 2016;31:421–7. doi: 10.1002/gps.4353. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Ling S, Yang Y, et al. Systematic hypothesis for post-stroke depression caused inflammation and neurotransmission and resultant on possible treatments. Neuroendocrinol Lett. 2014;35:104–9. [PubMed] [Google Scholar]
- 7.Spalletta G, Bossu P, Ciaramella A, et al. The etiology of poststroke depression: a review of the literature and a new hypothesis involving inflammatory cytokines. Mol Psychiatry. 2006;11:984–91. doi: 10.1038/sj.mp.4001879. [DOI] [PubMed] [Google Scholar]
- 8.Dziedzic T. Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev Neurother. 2015;15:523–31. doi: 10.1586/14737175.2015.1035712. [DOI] [PubMed] [Google Scholar]
- 9.Di Napoli M, Papa F, Bocola V. C-reactive protein in ischemic stroke: an independent prognostic factor. Stroke. 2001;32:917–24. doi: 10.1161/01.str.32.4.917. [DOI] [PubMed] [Google Scholar]
- 10.Boutin H, Pinborg LH. TSPO imaging in stroke: from animal models to human subjects. Clin Transl Imaging. 2015;3:423–35. [Google Scholar]
- 11.Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Rahola JG. Somatic drugs for psychiatric diseases: aspirin or simvastatin for depression? Curr Neuropharmacol. 2012;10:139–58. doi: 10.2174/157015912800604533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Köhler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–91. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 14.Kang JH, Kao LT, Lin HC, et al. Statin use increases the risk of depressive disorder in stroke patients: a population-based study. J Neurol Sci. 2015;348:89–93. doi: 10.1016/j.jns.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Kim JM, Stewart R, Kang HJ, et al. A prospective study of statin use and poststroke depression. J Clin Psychopharmacol. 2014;34:72–9. doi: 10.1097/JCP.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Han W, Gong D, et al. Hs-CRP in stroke: a meta-analysis. Clin Chim Acta. 2016;453:21–7. doi: 10.1016/j.cca.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt M, Schmidt SA, Sandegaard JL, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–90. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barber M, Fail M, Shields M, et al. Validity and reliability of estimating the scandinavian stroke scale score from medical records. Cerebrovasc Dis. 2004;17:224–7. doi: 10.1159/000075795. [DOI] [PubMed] [Google Scholar]
- 19.Pasco JA, Jacka FN, Williams LJ, et al. Clinical implications of the cytokine hypothesis of depression: the association between use of statins and aspirin and the risk of major depression. Psychother Psychosom. 2010;79:323–5. doi: 10.1159/000319530. [DOI] [PubMed] [Google Scholar]
- 20.Williams LJ, Pasco JA, Mohebbi M, et al. Statin and aspirin use and the risk of mood disorders among men. Int J Neuropsychopharmacol. 2016 Feb;:2. doi: 10.1093/ijnp/pyw008. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Köhler O, Petersen L, Mors O, et al. Inflammation and depression: combined use of selective serotonin reuptake inhibitors and NSAIDs or paracetamol and psychiatric outcomes. Brain Behav. 2015;5:e00338. doi: 10.1002/brb3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brouwers C, Christensen SB, Damen NL, et al. Antidepressant use and risk for mortality in 121,252 heart failure patients with or without a diagnosis of clinical depression. Int J Cardiol. 2016;203:867–73. doi: 10.1016/j.ijcard.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 23.Sarkar S, Chadda RK, Kumar N, et al. Anxiety and depression in patients with myocardial infarction: findings from a centre in India. Gen Hosp Psychiatry. 2012;34:160–6. doi: 10.1016/j.genhosppsych.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Müller N, Schwarz MJ, Dehning S, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–4. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- 25.Abbasi SH, Hosseini F, Modabbernia A, et al. Effect of celecoxib add-on treatment on symptoms and serum IL-6 concentrations in patients with major depressive disorder: randomized double-blind placebo-controlled study. J Affect Disord. 2012;141:308–14. doi: 10.1016/j.jad.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 26.Feng L, Tan CH, Merchant RA, et al. Association between depressive symptoms and use of HMG-CoA reductase inhibitors (statins), corticosteroids and histamine H(2) receptor antagonists in community-dwelling older persons: cross-sectional analysis of a population-based cohort. Drugs Aging. 2008;25:795–805. doi: 10.2165/00002512-200825090-00005. [DOI] [PubMed] [Google Scholar]
- 27.Maes M. Targeting cyclooxygenase-2 in depression is not a viable therapeutic approach and may even aggravate the pathophysiology underpinning depression. Metab Brain Dis. 2012;27:405–13. doi: 10.1007/s11011-012-9326-6. [DOI] [PubMed] [Google Scholar]
- 28.Müller N. The role of anti-inflammatory treatment in psychiatric disorders. Psychiatr Danub. 2013;25:292–8. [PubMed] [Google Scholar]
- 29.Aid S, Langenbach R, Bosetti F. Neuroinflammatory response to lipopolysaccharide is exacerbated in mice genetically deficient in cyclooxygenase-2. J Neuroinflammation. 2008;5:17. doi: 10.1186/1742-2094-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blais V, Turrin NP, Rivest S. Cyclooxygenase 2 (COX-2) inhibition increases the inflammatory response in the brain during systemic immune stimuli. J Neurochem. 2005;95:1563–74. doi: 10.1111/j.1471-4159.2005.03480.x. [DOI] [PubMed] [Google Scholar]
- 31.Palnum KH, Mehnert F, Andersen G, et al. Medical prophylaxis following hospitalization for ischemic stroke: age- and sex- related differences and relation to mortality. Cerebrovasc Dis. 2010;30:556–66. doi: 10.1159/000319030. [DOI] [PubMed] [Google Scholar]
- 32.Johnsen SP, Overvad K, Sorensen HT, et al. Predictive value of stroke and transient ischemic attack discharge diagnoses in the Danish National Registry of Patients. J Clin Epidemiol. 2002;55:602–7. doi: 10.1016/s0895-4356(02)00391-8. [DOI] [PubMed] [Google Scholar]