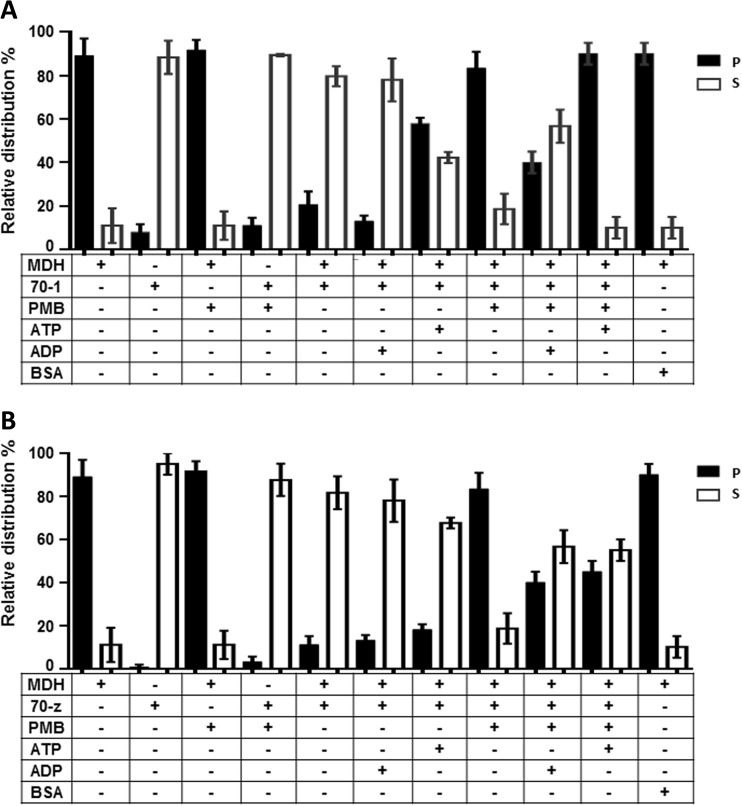
Fig. 5.
Polymyxin B suppresses chaperone function of PfHsp70-1and PfHsp70-z. The chaperone function of PfHsp70-1/PfHsp70-z was conducted by monitoring the heat-induced aggregation of MDH in vitro at 48 °C and quantitating the pellet (P) and soluble (S) fractions after heat exposure. The chaperone capabilities of PfHsp70-1 (a) and PfHsp70-z (b) were assessed by assessing the heat-induced aggregation of MDH in the presence of nucleotides and PMB, respectively. The activity of PfHsp70-1 and PfHsp70-z in the presence of PMB compared to the nucleotide-free state and ADP-bound state was statistically significant (p < 0.005), respectively. Standard deviations obtained from three replicate assays are shown