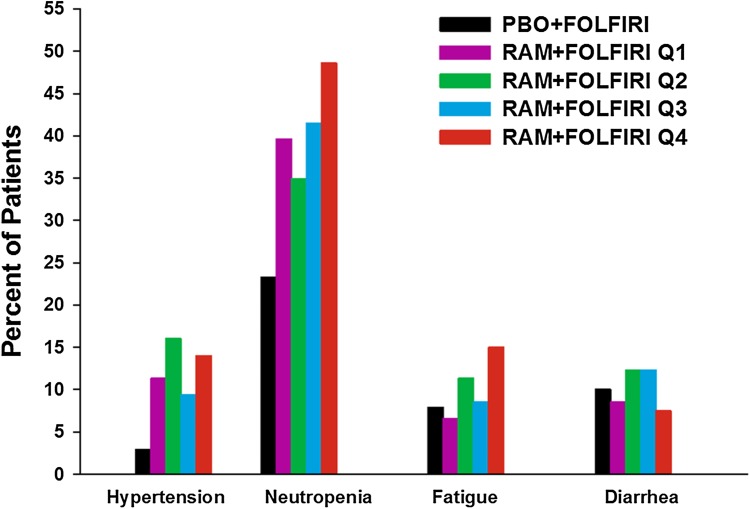
Fig. 4.
Incidence of Grade ≥3 treatment-emergent adverse events by ramucirumab Cmin,ss exposure quartile. There was only one reported Grade 4 hypertension event, nine reported Grade 4 diarrhea events, and no Grade 4 fatigue events. A total of 9.4% patients reported Grade 4 neutropenia. There were no Grade 5 events for all four safety endpoints. Treatment-emergent adverse events were graded by NCI-CTCAE v4.0. Neutropenia and fatigue are consolidated terms, meaning they are a composite term consisting of multiple related preferred terms based on Standardized Medical Dictionary for Regulatory Activities (MedDRA) Queries and medical review. Cmin,ss, minimum concentration at steady state; FOLFIRI, folinic acid, 5-fluorouracil, and irinotecan; NCI-CTCAE National Cancer Institute-Common Terminology Criteria for Adverse Events; PBO placebo; Q quartile; RAM ramucirumab