Table 3.
The relative activity of the recombinant AcCR to catalyze the oxidation of alcohols and reduction of ketones
| Substrate | Structure | Relative activity (%) | Substrate | Structure | Relative activity (%) |
|---|---|---|---|---|---|
| Isopropanol |
|
45.4 | |||
| 1-Phenethyl alcohol |
|
Nd | Acetophenone |
|
9.2 |
| 1-(4-Methylphenyl)ethanol |
|
16.6 | 4-Methylacetophenone |
|
16.5 |
| 2-Methoxyphenethyl alcohol |
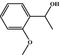
|
1.9 | 2-Methoxyacetophenone |
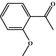
|
3.1 |
| 3-Methoxyphenethyl alcohol |
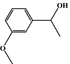
|
7.7 | 3-Methoxyacetophenone |
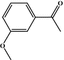
|
10.7 |
| 4-Methoxyphenethyl alcohol |
|
11.6 | 4-Methoxyacetophenone |
|
5.1 |
| 3,3-Dimethyl-2-butanol |
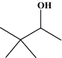
|
36.5 | 3,3-dimethyl-2-butanone |
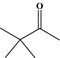
|
47.5 |
| 1-(Trimethylsilyl)-ethanol |
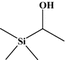
|
Nd | 1-(Trimethylsilyl)ethanone |
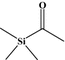
|
156.5 |
| 4-(Trimethylsilyl)-3-butyn-2-ol |
|
2.9 | 4-(Trimethylsilyl)-3-butyn-2-one |
|
114.4 |
| Methyl 3-hydroxybutyrate |
|
13.1 | Methyl acetoacetate |
|
81.1 |
| Ethyl 3-hydroxybutyrate |
|
6.2 | Ethyl acetoacetate |
|
52.1 |
| Ethyl (R)-4-chloro-3-hydroxybutyrate |
|
Nd | Ethyl-4-chloroacetoacetate |
|
120.6 |
| Ethyl (S)-4-chloro-3-hydroxybutyrate |
|
Nd | |||
| 1-(4-Fluorophenyl)ethanol |
|
55.1 | 4-Fluoroacetophenone |
|
85.1 |
| 1-(4-Chlorophenyl)ethanol |
|
52.3 | 4-Chloroacetophenone |
|
100.0 |
| 1-(4-Bromophenzyl) alcohol |
|
44.2 | 4-Bromoacetophenone |
|
117.1 |
| 1-(4-Nitrophenzyl) alcohol |
|
49.6 | 4-Nitroacetophenone |
|
98.7 |
| 2-Pentanol |
|
98.6 | 2-pentanone |
|
158.6 |
| 2- (R)-Octanol |
|
107.7 | 2-Octanone |
|
87.58 |
| 2- (S)-Octanol |
|
2.9 | |||
| Ethyl 2-hydroxy-4-phenylbutyrate |
|
2.3 | Ethyl 2- oxo-4-phenylbutyrate |
|
35.8 |
Reaction conditions: for oxidation reaction (a) 0.5 mM NAD+, 2 mL 50 mM Tris–HCl buffer (pH 8.5), 50 mM alcohol compound incubated at 45 °C for 5 min before adding 20 μL purified recombinant AcCR (about 0.008 mg the purified enzyme), recorded the changes of absorbance for 3 min at 45 °C; for reduction reaction (b) 0.25 mM NADH, 2 mL 50 mM citrate–phosphate buffer (pH 6.5), 50 mM carbonyl compound at 35 °C for 5 min before adding 20 μL purified recombinant AcCR (about 0.008 mg the purified enzyme) recorded the changes of absorbance for 3 min at 35 °C
