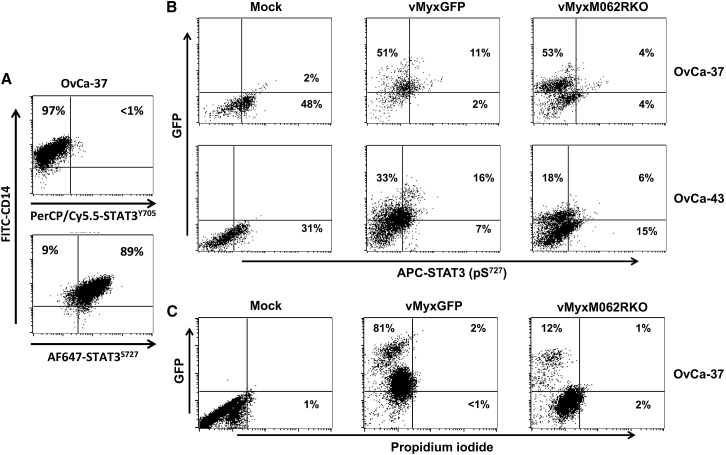
Figure 4.
MYXV Infection in OC Ascites-Associated CD14+ Monocytes Remodels the Intracellular Signaling Pathway
(A) OC patient ascites-associated CD14+ monocytes/macrophages display non-canonical STAT3 signaling. CD14+ monocytes were enriched from ascites fluid of patient (e.g., OvCa37) and tested for phosphorylation of STAT3 on both Y705 and S727 by flow cytometry. The signature of non-canonical STAT3 signaling is characterized by minimal phosphorylation at the Y705 site of STAT3. (B) MYXV infection in ascites-associated CD14+ monocytes causes inhibition of STAT3 phosphorylation at the Ser727 residue. CD14+ monocytes were enriched from patient-ascites fluid and tested for purity via flow cytometry. Monocytes were immediately mock treated and infected with WT or M062R-null MYXV at an MOI of 10 for 1 h; washing with PBS followed before the cells were cultured for 18 hr. Media was harvested for multiplex array (Figure 5). Cells were fixed and permeablized for intracellular staining as described in the Materials and Methods to examine the level of STAT3 phosphorylation at the Ser727 residue. (C) Infection by MYXV does not cause general change in cell viability in ascites CD14+ cells. Patient CD14+ ascites-associated monocytes were mock treated or infected with WT or M062R-null MYXV for 18 hr before the cells were stained with propidium iodide (PI). Live cells were gated to examine GFP (infection) and the presence of PI staining.