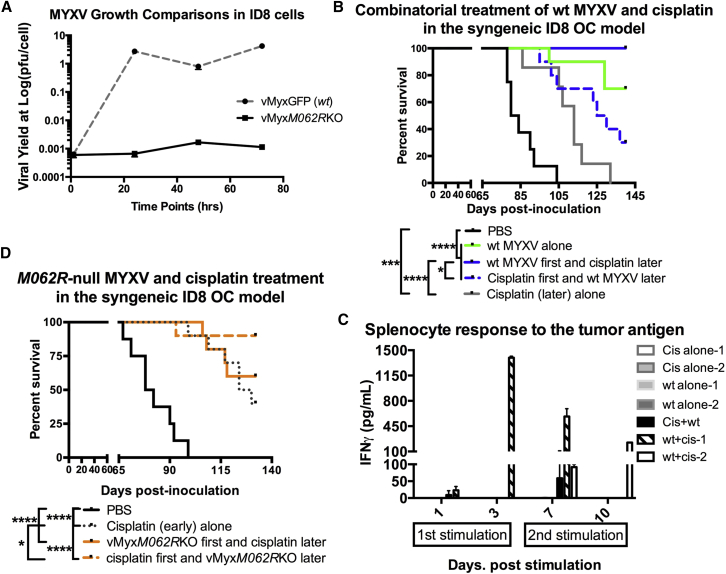
Figure 6.
Combinatorial Treatment of MYXV and Cisplatin for OC Disseminated Tumor in a Syngeneic Murine Model
(A) Multistep growth comparison of viral replication by WT and M062R null MYXV. In the murine OC cell line ID8, similar to most cells tested,47M062R-null MYXV underwent abortive infection. After infection by MYXV, ID8 cell lysates were harvested at given time points. Viral yields are quantified by titration. The error bar represents SEM, and the mean is calculated from the quantification in triplicate. Shown is a representative of two independent experiments. (B) MYXV pretreatment enhanced cisplatin effect in the ID8 OC dissemination model. At 7 days post-tumor-cell-injection, mice were either mock treated or treated with WT MYXV or cisplatin as described in the Materials and Methods. A second treatment occurred 5 days after the first treatment. Statistical analysis was carried out with the log-rank (Mantel-Cox) test (p < 0.0001) and the Gehan-Breslow-Wilcoxon test (p < 0.0001). Median survival (days) is calculated as follows: PBS (n = 8) 82 (days); cisplatin (late) only (n = 7), 113; WT MYXV only (n = 10), undefined; WT MYXV + cisplatin (n = 10), undefined; cisplatin first + WT MYXV later (n = 10), 127.5. *p < 0.05, ***p < 0.001, ****p < 0.0001. (C) Response to tumor antigen in mouse splenocytes from different treatments. Splenocytes harvested from mice treated with WT MYXV followed by cisplatin showed high levels of IFNγ when they were stimulated with ID8 tumor antigen. After 140 days, surviving mice from virus treated alone or combination treatment are euthanized to collect splenocytes; as controls, splenocytes were also harvested from mice with cisplatin alone at approximately 100 days for the test. Freshly harvested splenocytes were immediately stimulated with tumor cell lysate at day 0. Samples of supernatant were collected at days 1 and 3 post-stimulation. These splenocytes continued to be cultured, and at day 6, fresh media were added to include tumor cell lysate. On days 7 and 10, supernatant was collected to test IFNγ by ELISA. The error bar represents SD from the quantification in duplicate. Shown are representative mice of following groups: cisplatin alone (n = 2), WT MYXV alone (n = 2), cisplatin first plus WT MYXV later (n = 1), and WT MYXV first + cisplatin later (n = 2). (D) M062R null MYXV can be used in combination with cisplatin to achieve therapeutic benefit. Similar to (C), M062R-null MYXV was tested in the combinatorial treatment. Statistical significance was determined with the log-rank (Mantel-Cox) test (p < 0.0001), the log-rank test for trend (p < 0.0001), and the Gehan-Breslow-Wilcoxon test (p < 0.0001). Median survival (days) is calculated as following: PBS (n = 8) 80 (days); vMyxM062RKO first + cisplatin later (n = 10), undefined; Cisplatin first + vMyxM062RKO later (n = 10), undefined; cisplatin (early) alone (n = 10), 127. *p < 0.05, ****p < 0.001.