Abstract
Infection of boar-hunting dogs with Paragonimus westermani was investigated in Western Japan. Blood and rectal feces were collected from 441 dogs in the three districts (205 in Kinki, 131 in Chugoku and 105 in Shikoku District). In a screening ELISA for serum antibody against P. westermani antigen, 195 dogs (44.2%) showed positive reaction. In the 195 dogs, 8 dogs were found excreting P. westermani eggs after molecular analysis of fecal eggs, and additional 7 were identified serologically for the parasite infection because of their stronger reactivity against P. westermani antigen than against antigens of other species of Paragonimus. A spatial analysis showed that all of the P. westermani infections were found in Kinki and Chugoku Districts. In this area, dogs’ experience of being fed with raw boar meat showed high odds ratio (3.35) to the sero-positivity in the screening ELISA, and the frequency of such experiences was significantly higher in sero-positive dogs. While clear relationship was not obtained between predation of boars by dogs during hunting and their sero-positivity. Therefore, it is suggested that human activity of feeding with wild boar meat is the risk factor for P. westermani infection in boar-hunting dogs. Considering that hunting dogs could play as a major definitive host and maintain the present distribution of P. westermani in Western Japan, control measures for the infection in hunting dogs, such as prohibition of raw meat feeding and regular deworming, should be undertaken.
Keywords: dog, epidemiology, Japan, Paragonimus westermani, wild boar
Paragonimiasis is a typical food-borne parasitic disease caused by lung flukes of the genus Paragonimus [2, 3]. In Japan, three Paragonimus species, P. westermani, P. skrjabini miyazakii and P. ohirai, are recognized, and dogs can serve as the definitive host of all three species [11]. The first two species are known zoonotic parasites, with P. westermani predominating in human infection [18]. Approximately 50 cases of human paragonimiasis have been reported annually in Japan [16, 22, 29], however, how and where the parasite is maintained in nature are not clearly understood.
In Japan, there are two major routes of Paragonimus infections in humans; ingestion of second intermediate host, such as brackish water crabs, and ingestion of paratenic host, such as wild boars [23]. The latter route seems to be more important in current human cases [19,20,21, 23, 27, 31]. Recently, we encountered the infection of boar-hunting dogs in Miyazaki prefecture, Japan, with P. westermani [13, 25], and a subsequent survey revealed high sero-prevalence of paragonimiasis among boar-hunting dogs in the mountain range of central and southern Kyushu District [12]. Boar-hunting dogs are frequently fed with wild boar meat for feeding and training. Such dogs sometimes have a chance to predate boars during hunting. Thus, boar-hunting dogs are assumed to have higher chance of acquiring infection with P. westermani and could be an effective indicator for the epidemiological status of this parasite.
In this study, therefore, we expanded our study areas to other regions of western Japan, evaluated the current infection status of boar-hunting dogs in the region and elucidated the potential risk factors for the infection in the dogs.
MATERIALS AND METHODS
Serum and stool samples
With cooperation of hunters and local hunting associations, blood and stool samples (rectal feces) were collected from 441 boar-hunting dogs kept by 131 owners in Western Japan from 2009 to 2010 (Chugoku: 131 dogs/53 owners, Shikoku: 105/33 and Kinki: 205/45). Sera were stored at −30°C and feces at 4°C until use.
Positive control sera of dogs infected with P. westermani, P. s. miyazakii and P. ohirai
P. westermani-infected dog sera were obtained from 10 boar-hunting dogs that were kept by one owner in Miyazaki prefecture, Japan. The infection of the dogs was confirmed by PCR-sequencing of the second internal transcribed spacer region (ITS2) of nuclear ribosomal DNA [6, 7] of Paragonimus eggs excreted in the dogs’ feces. Serum from a dog experimentally infected with P. s. miyazakii and those from 3 dogs experimentally infected with P. ohirai were stored at −30°C for use as positive controls of corresponding species.
Adult worms of Paragonimus spp.
For antigen preparation and DNA references, adult worms of three Paragonimus species were obtained from dogs experimentally infected with metacercariae of P. westermani collected in Jilin, China; with those of P. s. miyazakii from Iwakuni, Yamaguchi Prefecture, Japan; and with those of P. ohirai from Miyazaki, Miyazaki Prefecture, Japan. Adult worms were stored at −30°C until use.
Antigen preparation for enzyme-linked immunosorbent assay (ELISA)
Somatic antigens of three Paragonimus species, P. westermani, P. s. miyazakii and P. ohirai, were prepared. Briefly, adult worms were cut into small pieces and sonicated using an electrical sonicator (BRANSON Sonifier 150, BRANSON, Danbury, CT, U.S.A.) in cold PBS on ice. The homogenate was kept at 4°C overnight with stirring and then centrifuged at 15,000 ×g at 4°C for 5 min. The supernatant was collected, and its protein concentration was measured by Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA, U.S.A.) with bovine serum albumin used as standard. The antigen solution was adjusted to 1 mg /ml and stored at −30°C until use. Somatic antigens of P. s. miyazakii and P. ohirai were prepared by the same method for that of P. westermani.
ELISA for Screening of Paragonimus infection
Screening ELISA for IgG against P. westermani antigen was performed as described previously on sera (×1,000 dilution) of boar-hunting dogs [12]. A cut-off value used was OD=0.2 as previously described.
Sero-estimation of P. westermani infection (serotyping ELISA)
For dogs that were sero-positive in the screening ELISA, P. westermani infection was further evaluated serologically, based on the difference of antibody response showing stronger signals against homologous antigen than heterologous ones [14, 32].
First, to develop criterion for serotyping, serum IgG levels against antigens of P. westermani (PwODv), P. s. miyazakii (PsmODv) and P. ohirai (PoODv) were evaluated for P. westermani positive control sera with dilution at 4,000× by the ELISA described above. Then, the differences of the mean values between PwODv and PsmODv and between PwODv and PoODv were calculated. Since P. westermani positive control sera showed higher values for PwODv than those for PsmODv and PoODv, we set up the serotyping criterion as following: P. westermani infection is suspected, if a test serum fulfilled both of the following two conditions: 1) PwODv of a test serum was higher than its PsmODv and PoODv. 2) Differences between OD values (PwODv-PsmODv and PwODv-PoODv) were higher than those of the positive control for P. westermani.
Finally, PwODv, PsmODv and PoODv were determined for each serum sample, and serotyping was performed following the above criterion.
Fecal egg examination
Feces of dogs that were sero-positive in the screening ELISA were examined for Paragonimus eggs by a modified Medical General Laboratory (MGL) method as described previously [8]. Briefly, 1 g of feces was mixed with 7 ml of water, passed through a wire mesh (pore size: 150 µm), mixed with 3 ml of ether and centrifuged at 190 ×g for 10 min. Then, the supernatant layer was collected and filtered through a nylon mesh having a pore size of 30 µm. The materials trapped on the mesh were washed off into water in a small container and examined under a stereomicroscope for the presence of the parasite egg.
For DNA analysis, eggs were collected by a simple sedimentation technique from 2 g of feces. After 3 serial sedimentation/decantation steps, the sediment was filtered through a nylon mesh (pore size: 30 µm). The sediment trapped on the mesh was transferred into water. Paragonimus eggs were retrieved individually under the stereomicroscope, fixed in 70% ethanol and stored at room temperature until use.
Molecular identification of Paragonimus species using egg DNA
Paragonimus egg was picked up individually under a stereomicroscope, transferred to PCR tube and mechanically crushed using a syringe needle. Each macerated egg was resuspended in 0.5 µl of Proteinase K digestion solution (10% Proteinase K, 100 mM Tris-HCl, 12.5 mM MEDTA, 150 mM NaCl and 1% SDS) and incubated at 55°C for 2 hr. The extracted genomic DNA was used as a template for PCR amplification of the ITS2 region using primers 3S (forward, 5′-CGC TGG ATC ACT CGG CTC GT-3′) and A28 (reverse, 5′-CCT GGT TAG TTT CTT TTC CTC CGC-3′) [4]. The products were purified using a QIAquick PCR Purification Kit (Qiagen, Hilden, Germany) and subjected to direct sequencing using a Big-Dye terminator cycle sequencing kit v3.1 (Applied Biosystems, Carlsbad, CA, U.S.A.) and a sequencer (Model 3100, Applied Biosystems). For species identification, the obtained sequences were subjected for Basic Local Alignment Search Tool (BLAST) homology search in the GenBank server of the National Center for Biotechnology Information [1]. The same region of genomic DNAs extracted from adult worms of P. westermani, P. s. miyazakii and P. ohirai was also sequenced for reference.
Questionnaire survey of owners of boar-hunting dogs
The following biological information relating to hunting background and the habitats of boar-hunting dogs was collected from the owners: 1) gender, 2) age, 3) breed (Japanese, European or mixed), 4) main hunting site, 5) history of eating fresh- or brackish-water crabs, 6) feeding with raw wild boar meat and its frequency and 7) history of nibbling on wild boars during hunting and its frequency.
Analysis of risk factors for sero-positivity
In this analysis, the study area was divided into two areas according to the detection of P. westermani: the Pw+ Area was defined as the area where P. westermani infection in dogs was detected, while the Pw− Area was defined as the area where P. westermani infection among dogs was not detected (Fig. 1). Statistical comparison was performed using Excel statistics 2010 software for Microsoft Excel 2010 for Windows (Social Survey Research Information Co., Ltd., Tokyo, Japan); P values of <0.05 were considered significant. For qualitative factors (gender, breed, predation of brackish water crabs or nibbling on wild boars, being fed with raw wild boar meat), a χ2 test was performed against sero-positivity, and the odds ratio (OR) was calculated. For quantitative factors (age, annual frequency of being fed with raw wild boar meat and annual frequency of nibbling on wild boar), median and interquartile range of each factor among dog groups (sero-positive and sero-negative) were calculated, and the Mann-Whitney U test was conducted between sero-positive and negative dogs for each factor.
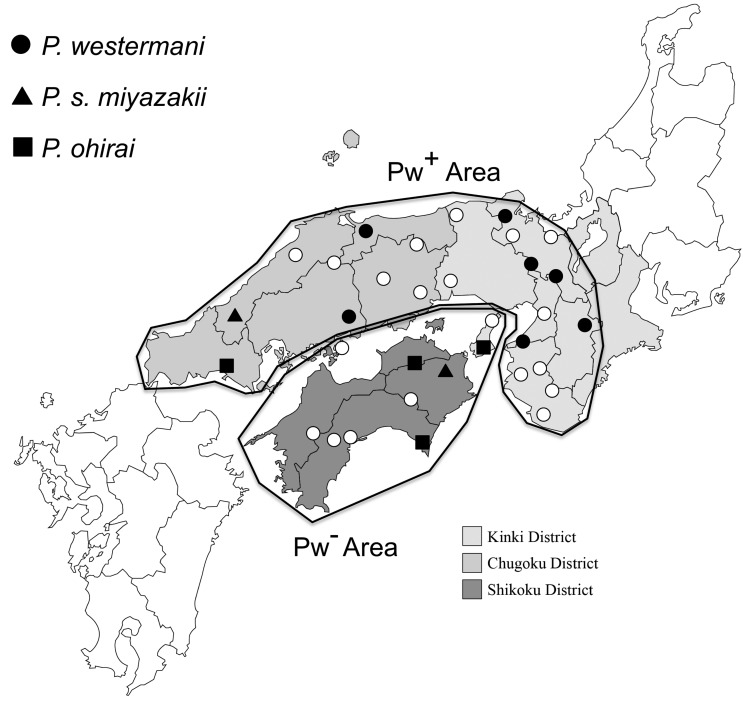
Fig. 1.
Location of main hunting sites and Paragonimus species detected. ○: site where infected species could not be determined, ●: site where P. westermani infection was detected, ▲: site where P. s. miyazakii infection was detected, ■: site where P. ohirai infection was detected, Pw+ Area: area where dogs released for hunting exhibited P. westermani infection, Pw− Area: area where dogs released for hunting did not exhibit P. westermani infection.
RESULTS
Seroprevalence of paragonimiasis in boar-hunting dogs
Among 441 serum samples collected from boar-hunting dogs kept by 131 owners, 195 (44.2%) were found to be sero-positive in the screening ELISA. The owners raised 1 to 12 dogs, with the mean of 3.4 per owner. Among the owners, 74.8% (98/131) harbored at least one sero-positive dog.
Fecal egg examination and molecular identification of Paragonimus species
Among 195 sero-positive dogs in the screening ELISA, Paragonimus eggs were found in the feces of 28 (14.4%) dogs by the modified MGL method, with the EPG (eggs per gram of feces) ranging from 1 to 169. However, DNA was not successfully extracted from the eggs collected by this method and thus, the simple sedimentation technique was applied to those 28 samples. Paragonimus eggs could be re-collected from only 17 samples, presumably reflecting low egg concentrations in the other 11 samples (in which only 1–12 eggs/g were found by the modified MGL method).
For the 17 simple-sedimentation-positive samples, one to three eggs, which species could not be discerned by morphology, were collected per sample. DNA was extracted from individual egg, and ITS2 sequence was determined for each egg. Even though multiple eggs were obtained from some samples, the sequences obtained from eggs in the same sample were identical with each other; thus, mixed infection was not detected in this analysis. In those 17 samples, P. westermani, P. s. miyazakii and P. ohirai were molecularly identified in 8, 3 and 6 samples, respectively.
Serotyping for estimating infecting species
Mean OD values of P. westermani positive control sera against the 3 species’ antigens in serotyping ELISA were 1.116, 0.892 and 0.754 for PwODv, PsmODv and PoODv, respectively. The differences in OD values of PwODv-PsmODv and PwODv-PoODv were 0.224 and 0.362, respectively. While Mean OD values of P. s. miyazakii positive control sera were 0.656, 1.133 and 0.710, and those of P. ohirai positive control sera were 0.948, 1.071 and 1.170 for PwODv, PsmODv and PoODv, respectively.
In the result of serotyping on 195 sero-positive dog sera in screening ELISA, sera of 11 dogs showed higher PwODv than its PsmODv and PoODv, with the median ratio of PwODv/PsmODv (minimum-maximum), 1.82 (1.35–2.70), and that of PwODv/PoODv, 2.39 (1.66–3.30). Therefore, P. westermani infection could be assigned on those 11 dogs that included 4 dogs excreting P. westermani eggs and 7 dogs showing P. westermani serotype but not excreting the parasite eggs.
Estimation of the current distribution of P. westermani
According to the owners, the dogs examined had been released primarily for hunting purposes at 33 sites. We therefore presumed that the dogs had been infected at the 33 sites, and used this information to estimate the current distribution of P. westermani. The number of dogs associated with each hunting site ranged from 2 to 35 (mean 13.4).
The fifteen dogs (8 with eggs and 7 with serology) that we defined as infected with P. westermani had been released at 7 of the 33 hunting sites. Since the 7 sites were located in Kinki and Chugoku Districts (excluding islands), we designated this area as the Pw+ Area in Fig. 1. The area composing of the other hunting sites was designated as the Pw− Area, where P. westermani egg or P. westermani serotype was not observed among surveyed dogs.
Dogs excreting P. s. miyazakii eggs had been released at 2 hunting sites; dogs excreting P. ohirai eggs had been released at 4 hunting sites (Fig. 1). No site yielded infection with more than one fluke worm.
Factors associated with sero-positivity in boar-hunting dogs
The numbers of dogs that had been released in the Pw+ and Pw− Areas were 295 and 146, respectively. In each area, differences in sero-prevalence among elements of qualitative variables were evaluated by χ2 tests (Table 1). A significant difference in sero-positivity was observed in the variable “being fed with raw wild boar meat”, with an OR of 3.35 for the “yes” group in the Pw+ Area. History of predation on boar during hunting did not show clear relationship to sero-positivity. None of qualitative variables in the Pw− Area showed a significant relationship to sero-positivity.
Table 1. Comparison of sero-prevalence against P. westermani antigen by elements of qualitative variables concerning dogs’ biological and hunting background in P. westermani detected (Pw+ Area) and non-detected (Pw− Area) areas.
| Variable | Element | Pw+ Area | Pw− Area | ||
|---|---|---|---|---|---|
| Prevalence (No. sero-positive/surveyed) |
Odds ratio | Prevalence (No. sero-positive/surveyed) |
Odds ratio | ||
| Gender | Male | 39.3 (66/168) | 0.90 | 51.9 (41/79) | 0.99 |
| Female | 41.7 (53/127) | 52.2 (35/67) | |||
| Breed | Japanese | 41.0 (71/173) | 0.83, 1.36, 1.64a) | 60.0 (33/55) | 2.00, 1.59, 0.79a) |
| European | 45.6 (26/57) | 42.9 (9/21) | |||
| Mixed | 33.9 (22/65) | 48.6 (34/70) | |||
| Predating brackish water crabs and/or crayfishes | Yes | None | NE | 50.0 (6/12) | 0.91 |
| No | 40.3 (119/295) | 52.2 (70/134) | |||
| Being fed with raw wild boar meat | Yes | 49.2 (97/197) | 3.35* | 55.0 (60/109) | 1.61 |
| No | 22.5 (22/98) | 46.2 (16/37) | |||
| Nibbling on wild boars | Yes | 46.7 (57/122) | 1.57 | 44.4 (20/45) | 0.64 |
| No | 35.8 (62/173) | 55.4 (56/101) | |||
a) Odds ratios for Japanese vs European, Japanese vs mixed and European vs mixed, respectively. *Significant difference (P<0.01) in the χ2 test. NE: not evaluated.
Medians of quantitative variables in sero-positive and sero-negative dogs in the two areas were compared in Table 2. In the Pw+ Area, sero-positive dogs had been fed with raw wild boar meat significantly more frequently than had sero-negative dogs. A significant difference was also observed in age. While, significant difference was not obtained in the frequency of predation on boar.
Table 2. Medians (with interquartile ranges) of quantitative variables in dogs that tested sero-positive and sero-negative against P. westermani antigen in P. westermani detected (Pw+ Area) and non-detected (Pw− Area) areas.
| Variable | Pw+ Area | Pw− Area | ||
|---|---|---|---|---|
| Sero-positive 119 dogs |
Negative 176 dogs |
Sero-positive 76 dogs |
Negative 70 dogs |
|
| Age (year) | 4 (2.5–7)a) | 3 (2–6) | 4 (3–7) | 4 (2–6) |
| Annual frequency of being fed with raw wild boar meat | 10 (5–48)b) | 3 (0–15) | 10 (4.5–20) | 10 (0–30) |
| Annual frequency of nibbling on wild boar | 0 (0–4.9) | 0 (0–10) | 0 (0–0.5) | 0 (0–0.8) |
Significant difference (a) P<0.05; b) P<0.01) in the Mann-Whitney U test (between sero-positive and sero-negative dogs).
DISCUSSION
Dogs being sero-positive in the screening ELISA were detected at high prevalence (44.2%) in the present study. The situation appeared to be similar to that reported previously in the Kyushu District [12]. For the screening ELISA, a crude somatic antigen made from P. westermani was used. Sensitivity of this method had been well confirmed in humans [24, 26], and thus, we supposed the method applicable for screening of Paragonimus infection in dogs as well. However, it is well known that being sero-positive against such antigen does not always indicate P. westermani infection because of antigenic similarity among Paragonimus spp. [5, 10, 15]. Indeed, in the present study, infection with three species of Paragonimus was found by molecular identification (ITS2 sequence from egg DNA) of fecal eggs, and only 8 dogs excreted P. westermani eggs. Because the number of dogs excreting P. westermani eggs was small, we have set a criterion for diagnosis of P. westermani infection by serology and tried to increase the number of potentially infected dogs with the parasite. In human patients, the OD values of sera infected with P. westermani were slightly higher in ELISA using P. westermani antigen than in that using P. s. miyazakii antigen, and vice versa [32]. The same trend showing stronger reactivity to the causative species antigen among several Paragonimus species was also reported [14]. Besides, further dilution of sera has been reported to potentially reduce such cross-reactivity [9]. In the present study, indeed, P. westermani positive control sera showed higher OD values against P. westermani antigen than against antigens of other two species, however, positive control sera for P. s. miyazakii and P. ohirai showed lower OD values against P. westermani antigen than against antigens of P. s. miyazakii and P. ohirai, respectively. Based on those observations, we designed the serotyping ELISA that revealed 11 P. westermani infected dogs, including 4 P. westermani egg-positive and 7 egg-negative dogs. Therefore, we found a total of 15 dogs (3.4%) potentially infected with P. westermani out of 441 dogs examined. There are few reports on canine paragonimiasis in Japan. A survey on stray dogs conducted in the mid-1950s reported a prevalence of less than 1% [30]. Comparing to this report, boar-hunting dogs in the present study showed higher prevalence with the parasite.
The 15 dogs potentially infected with P. westermani had been released for hunting in 7 sites located in Kinki and Chugoku Districts (the Pw+ Area). In this area, feeding of wild boar meat on dogs by their owners explained a significant relationship to sero-positivity, while history of predation on boar during hunting did not show clear relationship. Furthermore, sero-positive dogs fed more frequently with wild boar meat than sero-negative dogs. Therefore, it is suggested that human activity of feeding with wild boar meat is the risk factor for P. westermani infection in boar-hunting dogs. A significant difference observed in age between sero-positive and negative dogs in Pw+ Area could be reflecting that older dogs would have more chance of infection. This may explain that higher prevalence was observed in boar-hunting dogs in the present study than stray dogs in the previous study [30]. Boar-hunting dogs are commonly released in mountainous areas during hunting, and they have free defecation. Therefore, infected dogs could spread the parasite’s eggs elsewhere in hunting area and serve as a definitive host that maintains the lifecycle of P. westermani.
In recent years, human cases of P. westermani infection with history of wild boar meat consumption have been increasingly recognized [22]. Our result also suggests that wild boar meat can be an infection source for animals. More than 150,000 wild boars are hunted annually in Japan [17], and wild boar meat is now sold at local markets and via mail-order sales. Therefore, people consuming wild boar meat should pay high attention to that wild boar meat would be an infection source for P. westermani infection.
In the present study, although considerable human paragonimiasis due to P. westermani was reported in the Shikoku District during the 1971–1980 interval [28], no evidence was obtained for P. westermani distribution among hunting dogs released in that district. Although this study revealed that one and 4 dogs excreted P. s. miyazakii and P. ohirai eggs out of 105 dogs surveyed in this district, the number of dogs was not sufficient to deny the possibility that P. westermani is distributed in the Shikoku District. Therefore, further investigation on the occurrence of animal infection with P. westermani would be necessary. On the other hand, we did not detect P. westermani infection-suspected dogs in the west part of Chugoku District or the south part of Kinki District. Therefore, further investigation would be necessary on those regions as well.
In conclusion, P. westermani was found in dogs that had been released in sites in Kinki and Chugoku Districts. In this region, human activity of feeding with wild boar meat seemed to be the most important risk factor for P. westermani infection in boar-hunting dogs. Considering that hunting dogs could play an important role in maintaining the present distribution of P. westermani in Western Japan, control measures for the infection in hunting dogs, such as prohibition of raw meat feeding and regular deworming, should be undertaken.
FINANCIAL SUPPORT
Part of this work was supported by the Integrated Research Project for Human and Veterinary Medicine at the University of Miyazaki funded by the Ministry of Education, Culture, Sports, Science & Technology in Japan, and by JSPS KAKENHI Grant Number 21580377.
Acknowledgments
We are grateful to the staff of the Laboratory of Veterinary Parasitic Diseases, Department of Veterinary Sciences, Faculty of Agriculture, University of Miyazaki, for their valuable support. We also thank Mr. Masayuki Okuno, the local hunting association, and boar-hunters for providing dog specimens along with information related to this study. Lastly, we would like to send our special thanks to Dr. Yukifumi Nawa, Faculty of Medicine, Tropical Diseases Research Centre, Khon Kaen University, for his valuable advice and constructive discussion.
REFERENCES
- 1.Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J.1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402. doi: 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blair D., Agatsuma T., Wang W.2007. Paragonimiasis. pp. 117–150. In: World Class Parasites: volume 11. Food-Borne Parasitic Zoonoses. Fish and Plant-Borne Parasites. (Murrell, K. D. and Fried, B. eds.), Springer, New York. [Google Scholar]
- 3.Blair D., Xu Z. B., Agatsuma T.1999. Paragonimiasis and the genus Paragonimus. Adv. Parasitol. 42: 113–222. doi: 10.1016/S0065-308X(08)60149-9 [DOI] [PubMed] [Google Scholar]
- 4.Bowles J., Blair D., McManus D. P.1995. A molecular phylogeny of the human schistosomes. Mol. Phylogenet. Evol. 4: 103–109. doi: 10.1006/mpev.1995.1011 [DOI] [PubMed] [Google Scholar]
- 5.Dekumyoy P., Waikagul J., Eom K. S.1998. Human lung fluke Paragonimus heterotremus: differential diagnosis between Paragonimus heterotremus and Paragonimus westermani infections by EITB. Trop. Med. Int. Health 3: 52–56. doi: 10.1046/j.1365-3156.1998.00172.x [DOI] [PubMed] [Google Scholar]
- 6.Doanh P. N., Dung T., Thach D. T. C., Horii Y., Shinohara A., Nawa Y.2011. Human paragonimiasis in Viet Nam: epidemiological survey and identification of the responsible species by DNA sequencing of eggs in patients’ sputum. Parasitol. Int. 60: 534–537. doi: 10.1016/j.parint.2011.09.001 [DOI] [PubMed] [Google Scholar]
- 7.Doanh P. N., Shinohara A., Horii Y., Habe S., Nawa Y., The D. T., Le N. T.2007. Morphological and molecular identification of two Paragonimus spp., of which metacercariae concurrently found in a land crab, Potamiscus tannanti, collected in Yenbai Province, Vietnam. Parasitol. Res. 100: 1075–1082. doi: 10.1007/s00436-006-0411-9 [DOI] [PubMed] [Google Scholar]
- 8.Irie T., Yamaguchi Y., Sumen A., Habe S., Horii Y., Nonaka N.2015. Evaluation of the MGL method to detect Paragonimus eggs and its improvement. Parasitol. Res. 114: 4051–4058. doi: 10.1007/s00436-015-4632-7 [DOI] [PubMed] [Google Scholar]
- 9.Itoh M., Sato S.1990. Multi-dot enzyme-linked immunosorbent assay for serodiagnosis of trematodiasis. Southeast Asian J. Trop. Med. Public Health 21: 471–474. [PubMed] [Google Scholar]
- 10.Joo K. H., Ahn H., Chung M. S., Rim H. J.1989. Demonstration of species-specific and cross reactive components of Paragonimus westermani crude worm antigen by EITB. Korean J. Parasitol. 27: 9–14. doi: 10.3347/kjp.1989.27.1.9 [DOI] [PubMed] [Google Scholar]
- 11.Kawanaka M., Sugiyama H., Kato K.1999. Paragonimiasis acquired by eating boar meat: current status in Japan. Jpn. J. Infect. Dis. 52: 49. [PubMed] [Google Scholar]
- 12.Kirino Y., Nakano N., Doanh P. N., Nawa Y., Horii Y.2009. A seroepidemiological survey for paragonimosis among boar-hunting dogs in central and southern Kyushu, Japan. Vet. Parasitol. 161: 335–338. doi: 10.1016/j.vetpar.2009.01.011 [DOI] [PubMed] [Google Scholar]
- 13.Kirino Y., Nakano N., Hagio M., Hidaka Y., Nakamura-Uchiyama F., Nawa Y., Horii Y.2008. Infection of a group of boar-hunting dogs with Paragonimus westermani in Miyazaki Prefecture, Japan. Vet. Parasitol. 158: 376–379. doi: 10.1016/j.vetpar.2008.09.017 [DOI] [PubMed] [Google Scholar]
- 14.Knobloch J.1984. Application of different Paragonimus antigens to immunodiagnosis of human lung fluke infection. Arzneimittelforschung 349B: 1208–1210. [PubMed] [Google Scholar]
- 15.Mannoji N.1952. Further notes on the lung-fluke, Paragonimus ohirai Miyazaki, 1939. Part iii. Immunological studies of the lung-fluke, P. ohirai Miyazaki, 1939. Acta Med. 22: 1197–1224(in Japanese with English Summary). [Google Scholar]
- 16.Maruyama H., Nawa Y.2007. Paragonimus. Jpn. J. Chest Dis. 66: 269–275(in Japanese). [Google Scholar]
- 17.Ministry of the Environment Government of Japan2013. Statistics of Birds and Animals in 2013. https://www.env.go.jp/nature/choju/docs/docs2/h25/06h25tou.html [accessed April 20, 2016] (in Japanese).
- 18.Miyazaki I.1991. Paragonimiasis. pp. 76–146. In: An Illustrated Book of Helminthic Zoonosis (Miyazaki, I. ed.) International Medical Foundation of Japan, Tokyo. [Google Scholar]
- 19.Miyazaki I., Habe S.1976. A newly recognized mode of human infection with the lung fluke, Paragonimus westermani (Kerbert 1878). J. Parasitol. 62: 646–648. doi: 10.2307/3279438 [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki I., Hirose H.1976. Immature lung flukes first found in the muscle of the wild boar in Japan. J. Parasitol. 62: 836–837. doi: 10.2307/3278977 [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki I., Terasaki K., Iwata K.1978. Natural infection of muscle of wild boars in Japan by immature Paragonimus westermani (Kerbert 1878). J. Parasitol. 64: 559–560. doi: 10.2307/3279814 [DOI] [PubMed] [Google Scholar]
- 22.Nagayasu E., Yoshida A., Hombu A., Horii Y., Maruyama H.2015. Paragonimiasis in Japan: a twelve-year retrospective case review (2001-2012). Intern. Med. 54: 179–186. doi: 10.2169/internalmedicine.54.1733 [DOI] [PubMed] [Google Scholar]
- 23.Nakamura-Uchiyama F., Mukae H., Nawa Y.2002. Paragonimiasis: a Japanese perspective. Clin. Chest Med. 23: 409–420. doi: 10.1016/S0272-5231(01)00006-5 [DOI] [PubMed] [Google Scholar]
- 24.Nakamura-Uchiyama F., Onah D. N., Nawa Y.2001. Clinical features of paragonimiasis cases recently found in japan: parasite-specific immunoglobulin M and G antibody classes. Clin. Infect. Dis. 32: e171–e175. doi: 10.1086/320750 [DOI] [PubMed] [Google Scholar]
- 25.Nakano N., Kirino Y., Uchida K., Nakamura-Uchiyama F., Nawa Y., Horii Y.2009. Large-group infection of boar-hunting dogs with Paragonimus westermani in Miyazaki Prefecture, Japan, with special reference to a case of sudden death due to bilateral pneumothorax. J. Vet. Med. Sci. 71: 657–660. doi: 10.1292/jvms.71.657 [DOI] [PubMed] [Google Scholar]
- 26.Nawa Y.1998. Histopathological and immunological diagnosis for parasitic zoonoses. pp. 39–52. In: Host Response to International Parasitic Zoonoses (Ishikura, H., Aikawa, M., Itakura, H. and Kikuchi, K. eds.), Springer-Japan, Tokyo. [Google Scholar]
- 27.Nawa Y., Nakamura-Uchiyama F.2005. Paragonimus and paragonimiasis in Japan. pp. 125–131. In: Food-borne Helminthiasis in Asia, Asian Parasitology vol. 1 (Arizono, N., Chai, J. Y., Nawa, Y. and Takahashi, Y. eds.), AAA committee/Federation of Asian Parasitologists, Chiba. [Google Scholar]
- 28.Nishida H., Shibahara T.1999. Epidemiology of Paragonimus pp. 189–203. In: Progress of Medical Parasitology in Japan vol. vii. (Otsuru, M., Kamegai, S. and Hayashi, S. eds.), Meguro Parasitological Museum, Tokyo (in Japanese). [Google Scholar]
- 29.Sugiyama H.2010. Food-borne parasitic infection as food poisoning. Jpn. J. Food Microbiol. 27: 1–7(in Japanese). doi: 10.5803/jsfm.27.1 [DOI] [Google Scholar]
- 30.Tanaka T., Sei Y., Kugita Y.1955. Natural infection of dogs with the Paragonimus in Nagasaki city. Endemic Dis. Bull. Nagasaki Univ. 4: 1491–1494(in Japanese). [Google Scholar]
- 31.Uchiyama F., Morimoto Y., Nawa Y.1999. Re-emergence of paragonimiasis in Kyushu, Japan. Southeast Asian J. Trop. Med. Public Health 30: 686–691. [PubMed] [Google Scholar]
- 32.Waikagul J.1989. Serodiagnosis of paragonimiasis by enzyme-linked immunosorbent assay and immunoelectrophoresis. Southeast Asian J. Trop. Med. Public Health 20: 243–251. [PubMed] [Google Scholar]