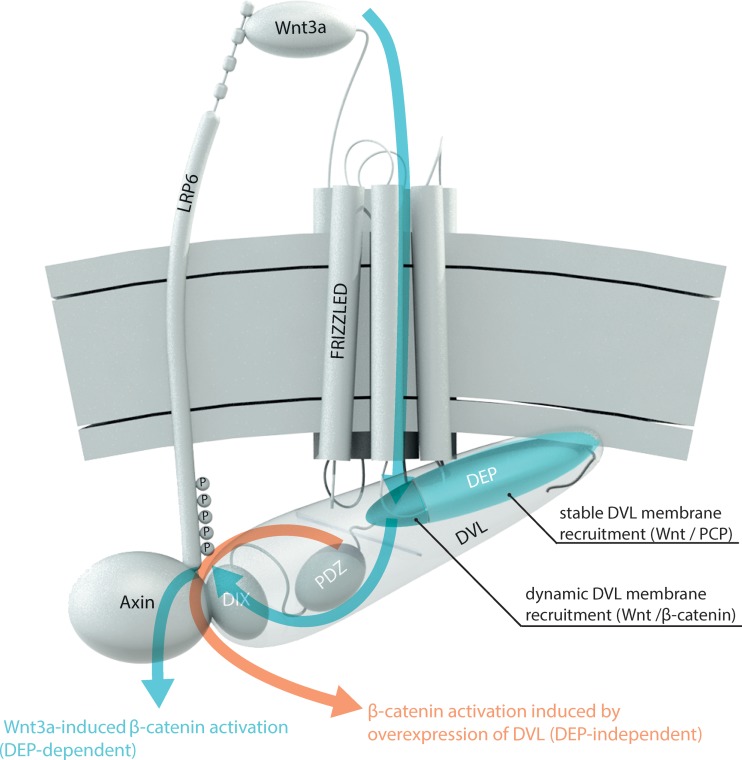
FIG 6.
Model of the role of the Dishevelled DEP domain in Wnt/β-catenin signal transduction. Signal transduction is initiated by the interaction of Wnt3a with the extracellular part of LRP6 and Frizzled. DVL is recruited to the membrane and interacts with FZD. The N-terminal part of the DVL DEP domain (dark blue) is necessary for dynamic DVL membrane recruitment to Frizzled after Wnt3a treatment. Other regions of the DEP domain (light blue) that are important for the stable interaction of DVL with FZD and/or the plasma membrane and that are required for the function of DVL in the Wnt/PCP pathway are indispensable in this process. Via its DIX domain, DVL interacts with axin, which brings axin to the membrane complex and leads to LRP6 phosphorylation and downstream Wnt/β-catenin signaling. This Wnt3a-induced β-catenin signal transduction is indicated by blue arrows. Overexpression of DVL, commonly used in the experiments in the Wnt field, can bypass the requirement for the DEP domain in the Wnt/β-catenin pathway by nucleating endogenous DVL and axin (via the DIX domain) and triggering Wnt ligand-independent β-catenin activation. This pathway is indicated by an orange arrow.