Fig. 5.
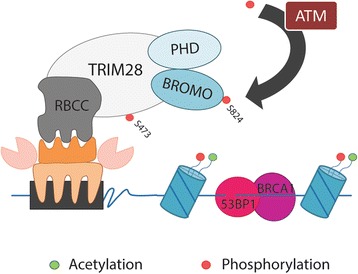
ATM-mediated phosphorylation of TRIM28 upon DNA double-strand break leads to destabilization of heterochromatin and enhances the accession of DNA repair machinery to the damaged site. In response to DNA damage, PI3-Kinase family members, like ATM, phosphorylate TRIM28 at serine 824 (near BROMO domain) and serine 473 (near HP1 binding domain). Serine 824 phosphorylation leads to abrogated NuRD and SETDB1 recruitment, and therefore halts heterochromatin compaction [47, 51]. Phosphorylation of TRIM28 at serine 473 attenuates its binding to the HP1 family proteins, and inhibits its transcriptional repression activity to KRAB-ZNFs target genes. Altogether, phosphorylation of TRIM28 results in chromatin relaxation that enhances the accession of DNA repair machinery (BRCA1 and 53BP1 proteins) to the damaged site [50]. BRCA1 – Breast Cancer 1; 53BP1 – p53 Binding Protein 1
