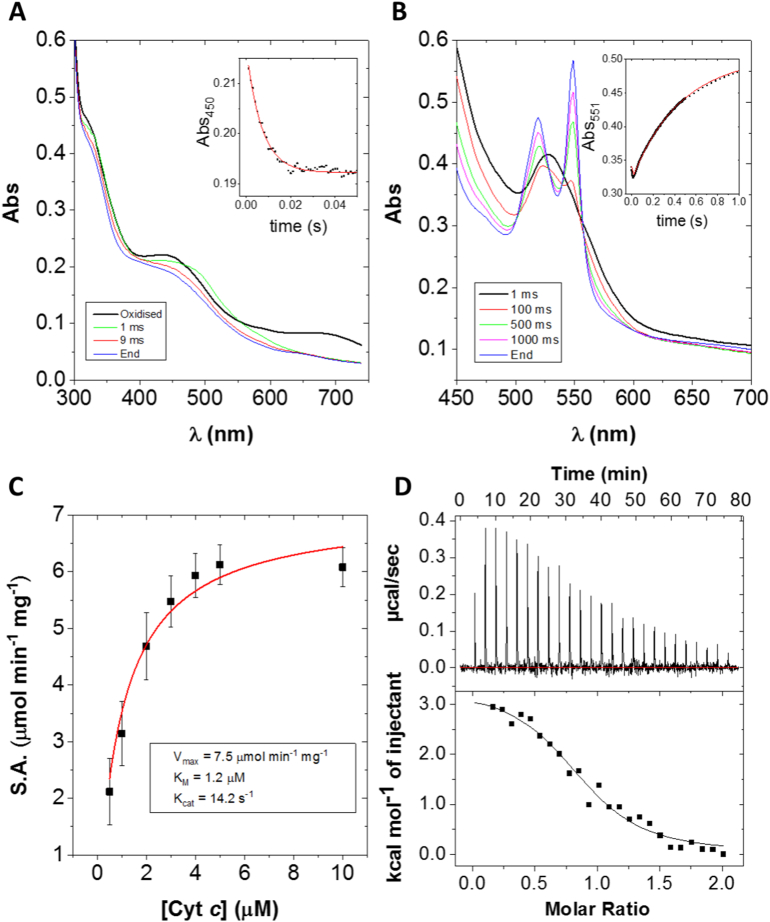
Fig. 3.
Results of measurements of kinetic and thermodynamic parameters of the AioBF108A mutant. A) Reductive half-reaction of F108A with arsenite. Inset: change in absorbance at 450 nm with single exponential fit (red line). B) Reduction of cytochrome c by F108A and arsenite. Inset: change in absorbance at 551 nm with triple exponential fit (red line). Stopped-flow results are the averages of 4–6 repeats with one enzyme preparation. C) Steady-state kinetics of cytochrome c and F108A with excess arsenite fit with the Michaelis-Menten model (red line). Results are the average of three separate enzyme preparations. S.A. stands for specific activity. D) Upper panel: ITC raw thermogram of F108A titrated against cytochrome c. Lower panel: Heats integrated with respect to time and plotted against molar ratio with a 1:1 binding fit (black line). Results are the average of three separate enzyme preparations.