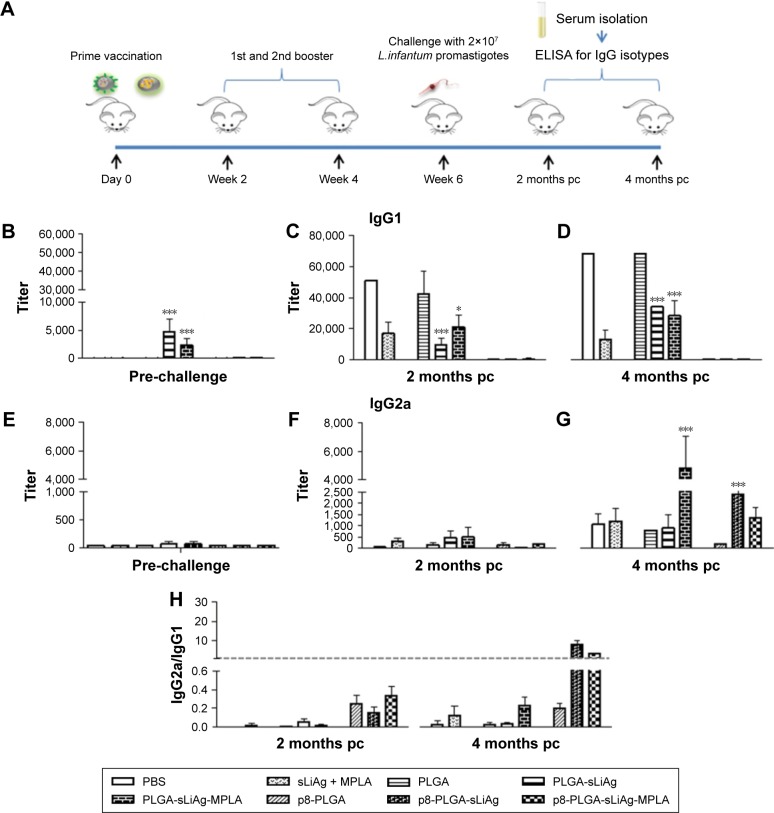
Figure 2.
Specific anti-sLiAg antibodies raised in vaccinated–infected BALB/c mice. (A) Groups of mice (n=5) were vaccinated with PBS, sLiAg + MPLA, PLGA, PLGA-sLiAg, PLGA-sLiAg-MPLA, p8-PLGA, p8-PLGA-sLiAg or p8-PLGA-sLiAg-MPLA three times with 2-week intervals. Two weeks after the second booster vaccination, mice were challenged with 2×107 stationary-phase promastigotes of Leishmania infantum. (B and E) Two weeks after the last vaccination and (C, D, F and G) 2 and 4 months post-challenge, specific anti-sLiAg IgG1 and IgG2a antibodies were determined in mice serum using ELISA. Samples were run in duplicates. (H) IgG2a-to-IgG1 ratio. Results are expressed as titer ± SD, that is, the maximum dilution in which sample OD > cut-off OD. Significant differences between mice vaccinated with the multifunctionalized NPs and PBS control group are indicated by asterisks: *P<0.05 or ***P<0.001.
Abbreviations: sLiAg, soluble Leishmania infantum antigens; PBS, phosphate-buffered saline; MPLA, monophosphoryl lipid A; PLGA, poly(D,L-lactide-co-glycolide); p8, eight-amino-acid peptide; NPs, nanoparticles; pc, post-challenge; ELISA, enzyme-linked immunosorbent assay; OD, optical density.