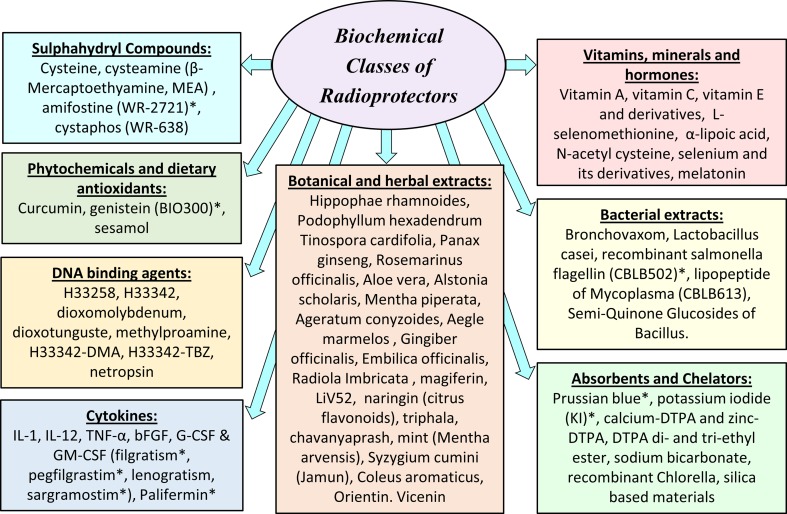
Fig. 1.
Schematic representation of various classes of radioprotectors citing most studied compounds. Asterisks indicate agents approved or under considerations for clinical use as follows: Amifostine (Ethyol) is specifically approved by FDA as a radioprotector that prevents cumulative normal tissues toxicity associated with cancer treatments, and offers significant reduction of radiation-induced xerostomia in head and neck radiotherapy patients. Genistein (BIO300) has currently an investigational new drug (IND) status as radioprotector of normal tissues to prevent acute radiation syndromes. While FDA has approved Neupogen (filgratism), Neulasta (pegfilgrastim) and Leukine (sargramostim) to ameliorate neutropenia induced by cancer treatment, these compounds are currently under investigation as radiation countermeasure agents. Palifermin (Kepivance) is an FDA-approved recombinant derivative of human keratinocyte growth factor (KGF) that is used to treat oral mucositis in patient undergoing hematopoietic stem cell transplantation. Recombinant salmonella flagellin CBLB502 (Entilimod) is FDA approved as off-label drug that can be used during nuclear or radiological accidents to protect against acute radiation syndromes. Prussian blue (Radiogardase) and potassium iodide (KI) are FDA-approved decorporating agents to increase the rate of elimination of radionuclides in internal contamination