Figure 6.
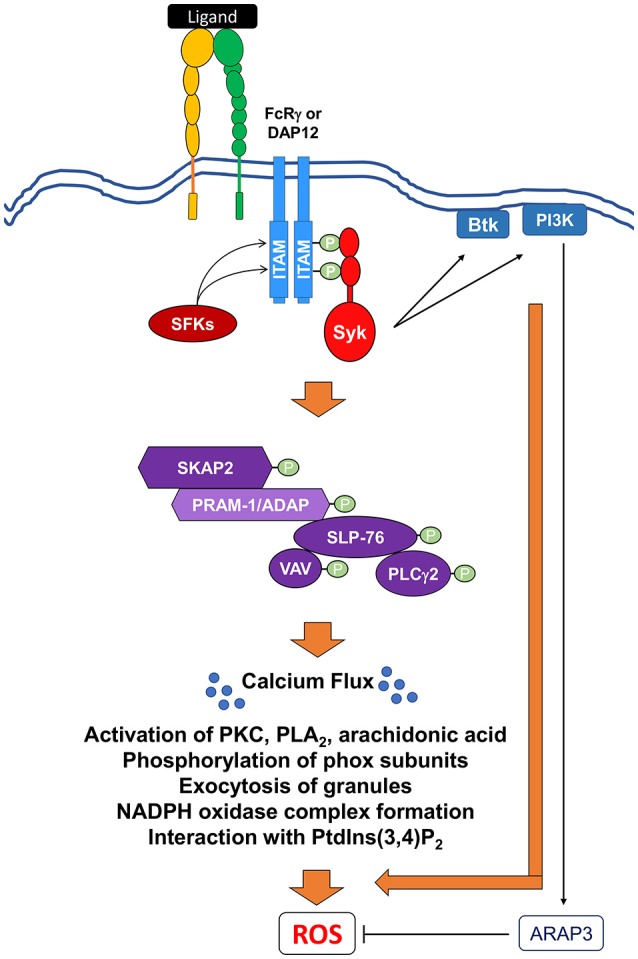
Signaling pathways mediating integrin-induced NADPH oxidase activation. Ligation and crosslinking of integrin receptors leads to the phosphorylation of the ITAM-containing proteins, DAP12 and FcRγ, by (SFKs), resulting in the recruitment and the tyrosine phosphorylation of the Src homology domain of Syk. Activated Syk can then act to recruit and activate Bruton's tyrosine kinase (Btk) and class I phosphoinositide 3-kinase (PI3K). A class I PI3K effector, ARAP3, has been shown to negatively regulate ROS production. Syk also induces the activation of SH2-domain-containing leukocyte protein of 76 kDa (SLP76) to form a multi-protein signaling complex. This SLP76 complex can then recruit and activate downstream effectors proteins like SKAP2, SLP76, the Vav GEF family, and PLCγ2. Activation of this complex leads to further downstream effectors resulting in the release of intracellular calcium stores (Ca2+ flux) and ultimate ROS production. Activation of these proximal signaling molecules lead to exocytosis of granules, activation of various PKC family members, phospholipase A2 (PLA2), and release of arachidonic acid, a lipid messenger. All of these secondary messengers are required for phosphorylation of phox subunits, formation of NADPH oxidase, and interaction with phosphatidylinositol 3,4-biphosphate (PtdIns(3,4)P2).
