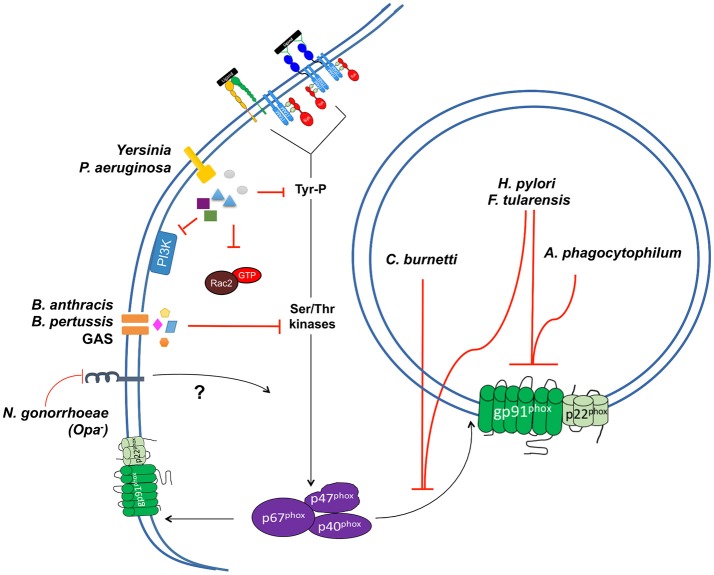
Figure 7.
Mechanisms of NADPH oxidase inhibition by bacterial pathogens in PMNs. Several bacterial pathogens employ mechanisms to interfere with the activation and/or localization of the NADPH complex of PMNs. These include strategies to prevent oxidative burst in the phagosomal compartment. Three pathogens, F. tularensis, A. phagocytophilum, and H. pylori, exclude one or both components of the cytb558 complex from the phagosomal membrane. Three pathogens, F. tularensis H. pylori, and C. burnetti, exclude or prevent p67phox/p40phox/p47phox from binding to the phagosomal membrane. A number of extracellular pathogens also employ mechanisms to inhibit oxidative burst. P. aeruginosa inhibits the oxidative burst of PMNs through the activities of two T3SS effectors, ExoS and ExoT. Both effectors inhibit activation of PI3K signaling pathways upstream of p67phox/p40phox/p47phox activation. The pathogenic Yersinia sp. inhibit respiratory burst in PMNs, though their activities have been largely modeled in other phagocytic cell types. Y. pseudotuberuclosis translocates the effector protein YopE through a T3SS to block activation of Rac in HL-60 cells. Y. pseudotuberculosis also translocates another T3SS effector protein, YopH, which interferes with oxidative burst in macrophages. The effects of YopH on oxidative burst have not been examined in PMNs; it dismantles the SLP-76/SKAP2 signal transduction pathway in these cells, suggesting that interference of this pathway in PMNs could prevent oxidative burst. Three pathogens, B. anthracis, B. pertussis, and Group A Streptococcus (GAS), also secrete toxins into PMNs that interfere with signaling pathways required for oxidative burst. Finally, strains of N. gonorrhoeae lacking opacity-associated proteins do not activate oxidative burst in PMNs, though the mechanism by which this occurs remains unclear. It is hypothesized that the failure of opacity-negative strains to engage CEACAM receptors could result in a failure to stimulate kinase signaling upstream of p47phox activation. Alternatively, it is possible that opacity-negative strains may actively block trafficking of NADPH oxidase components to membrane sites. Additionally, three other pathogens, L. monocytogenes, S. typhimurium, and V. parahaemolyticus, are capable of inhibiting the oxidative burst in cultured cells; however, their effects on neutrophils have not been examined in detail.