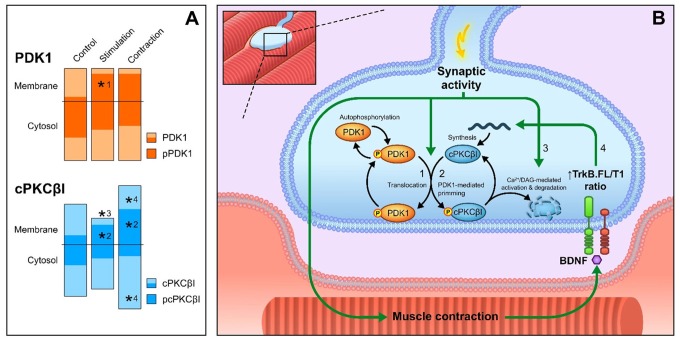
Figure 7.
Overview of PDK1 function and cPKCβI phosphorylation at the NMJ. (A) Summary of the results of this work. Columns represent the total protein levels of PDK1 (in orange), pPDK1 (in dark orange), cPKCβI (in blue) and pcPKCβI (in dark blue) in the membrane and cytosol compartments (indicated with a horizontal line) *p < 0.05. (B) Proposed model of the action of PDK1 on cPKCβI phosphorylation at the NMJ. Total PDK1 protein and phosphorylation levels are maintained throughout all conditions. (#1) Synaptic activity induces the translocation of pPDK1 to the membrane. Consistent with the increased pPDK1 in the membrane, synaptic activity also (#2) increases the phosphorylation of cPKCβI in the same compartment. Once catalytically competent, pcPKCβI activation through synaptic activity (#3) reduces the total amount of cPKCβI in the membrane due to an increase in its activation-induced degradation. Muscle contraction (#4) increases the total amount of cPKCβI in both cytosol and membrane, suggesting an activation of its synthesis. In previous work, we determined that this enhancement is retrogradely regulated through BDNF/TrkB signaling (Hurtado et al., 2017). After muscle contraction, PDK1 activity remains unaltered and, therefore, pcPKCβI levels increase in concordance with total cPKCβI (#2). Abbreviations: phosphoinositide-dependent kinase 1; pPDK1, phosphorylated phosphoinositide-dependent kinase 1; cPKCβI, conventional protein kinase C βI; pPKCβI, phosphorylated conventional protein kinase C βI; BDNF, brain-derived neurotrophic factor; TrkB.FL, tropomyosin-related kinase B full-length, TrkB.T1, tropomyosin-related kinase B truncated isoform 1.