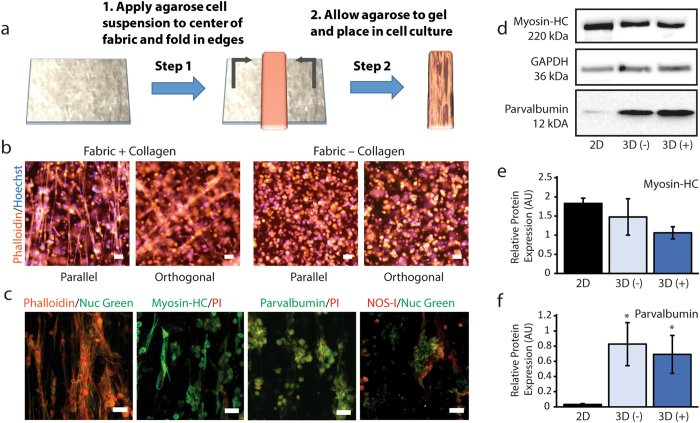
Figure 3.
Incorporation of collagen networks in 3D hydrogel scaffolds supports cell alignment and differentiation. (a) Dry fabrics can be readily incorporated into commonly-used non-cell-adherent hydrogels such as agarose. (b) Low magnification epifluorescence images of actin-phalloidin and Hoechst nuclear staining demonstrate that collagen-doped fabrics promote large-scale alignment of agarose-encapsulated C2C12 cells. Cell alignment and growth is not observed for control fabrics that do not contain collagen. Arrows indicate direction of fabric alignment. (c) High-resolution confocal maximum intensity projections show details of aligned fascicle-like structures. Positioning of cell nuclei and interconnected cytoskeleton structures are indicative of myotube formation. Myotubes and fascicle-like structures are not observed for cells cultured with control fabrics. C2C12 cells growing along collagen networks within the hydrogels display immunofluorescence for skeletal muscle cell differentiation markers including myosin heavy chain, parvalbumin and NOS-I. All scale bars, 50 µm. (d) Representative Western blots for myosin heavy chain, parvalbumin and GAPDH for cells grown in 2D on tissue culture plastic, within agarose containing 3D control fabrics (3D (−)) and within agarose containing 3D collagen fabrics (3D (+)). Images are cropped from the original full blots with adjustment of brightness and contrast to ensure that faint bands are visible. Unprocessed Western blot images are available in Supplementary Figure 7. Levels of myosin heavy chain (e) and parvalbumin (f) relative to GAPDH (AU; Arbitrary Units) were compared across culture conditions. Significant differences of p < 0.05 by one-way ANOVA on ranks and Dunnett’s multiple comparison test with respect to the 2D control group are indicated by *. Densitometry data are available in Supplementary Table 1.