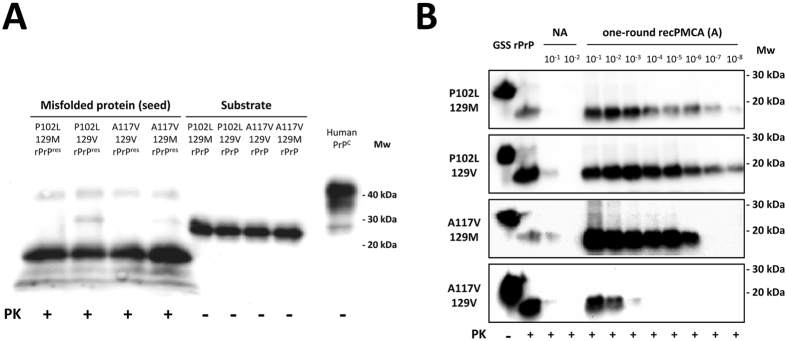
Figure 3.
In vitro propagation ability study of in vitro generated GSS-associated misfolded rec-PrPs. (A) Western blot of the four in vitro generated GSS-associated misfolded rec-PrPs used as seed after PK digestion (25 µg/ml) (rPrPres) and the four undigested substrates containing the corresponding GSS-associated rec-PrPs (rPrP). Note the signal from both, seeds and substrates were similar. The concentration of the recombinant proteins that comprised the different substrates was also adjusted using the BCA Protein assay. (B) Western blots of amplified samples subjected to a single 48 h round recPMCA (A). Each GSS-associated misfolded rec-PrPs was subjected to serial dilutions in the substrate containing their respective rec-PrPs (same substitution and polymorphism at codon 129). The dilutions employed ranged from 10−1 to 10−8. 10−1 and 10−2 dilutions without undergoing recPMCA were used as controls of the signal derived from the initial seed prior to amplification (NA). All samples were digested with 25 µg/ml of PK. The seeds containing the P102L substitution with either polymorphic variants at codon 129 were able to amplify until at least 10−8 dilution. However, the misfolded A117V-129M rec-PrP showed 100 times less in vitro propagation ability, amplifying up to a 10−6 dilution. Moreover, misfolded A117V-129V rec-PrP amplified just up to a 10−3 dilution. PK-untreated recombinant proteins were used as control (GSS rPrP). 3F4 monoclonal antibody (1:10,000) was used for visualisation. Mw: Molecular weight.