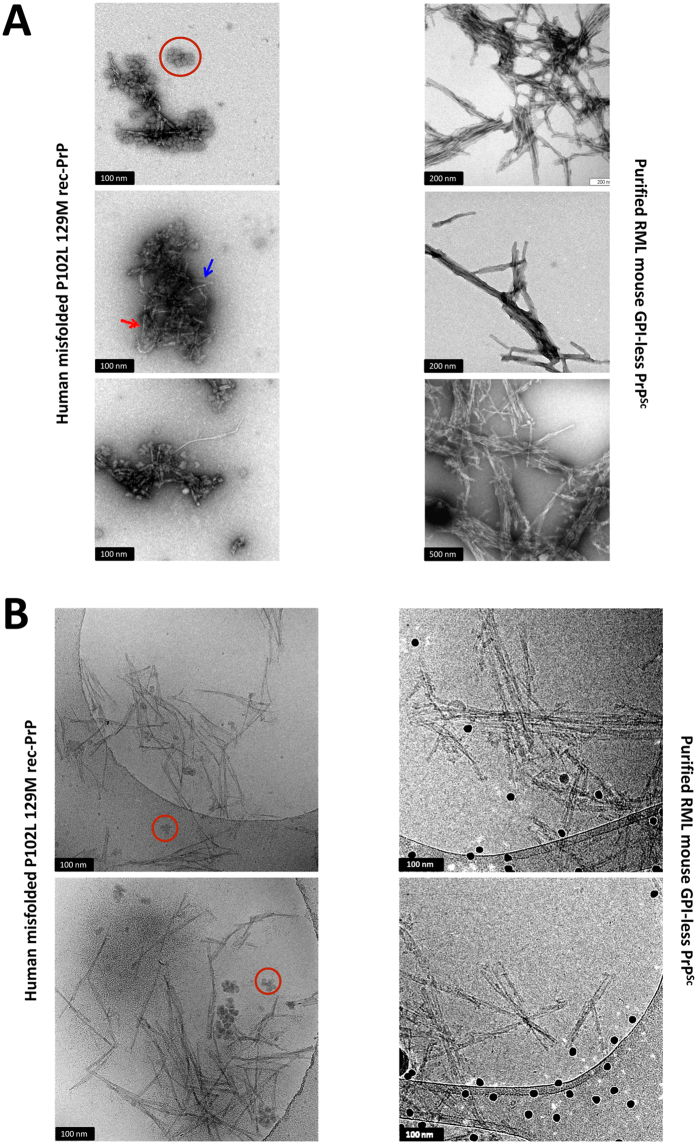
Figure 5.
Electron microscopy analysis of misfolded P120L-129M rec-PrP. (A) Negative stain TEM images of misfolded P102L-129M rec-PrP. Samples were deposited in freshly glow-discharged carbon-coated gold grids and stained with 5% uranyl acetate. Short rod-like structures (left images), exhibit a close resemblance to those observed in RML GPI-anchorless PrPSc (right images). In both cases images show bundles of laterally associated fibrils (“rods”); in many instances, the individual fibrils that by association make up the rods can be discerned. As previously reported in many previous studies, these fibrils are ~10 nm wide and in turn composed of two intertwined individual protofilaments (an example is signalled with a red arrow). At times, the two individual protofilaments run parallel in a short stretch of the fibril (blue arrow). Amorphous material corresponding likely to fibrils shattered during the PMCA was also present. Rosette-like structures made of glycogen, present as an impurity in the RNA used as a co-factor for generation of the recombinant PrP are also observed (red circles). (B) Cryo-EM images of the misfolded P102L-129M rec-PrP. The sample was concentrated 100 times by sedimentation after PK digestion (100 µg/ml). Short rod-like structures of 100 to 200 nm in length are present. Rosette-like structures containing glycogen are also shown (red circles). As in TEM images, fibrils in the misfolded rec-PrP preparations showed a high resemblance to those observed in the purified PrPSc sample from brains of GPI-anchorless transgenic mice inoculated with RML prion strain (right images): in both cases, the basic element is a ~10 nm fibril made up of 2 intertwined protofilaments. In images of GPI-anchorless PrPSc (right), dark dots are 15 nm fiducial gold particles.