Figure 1.
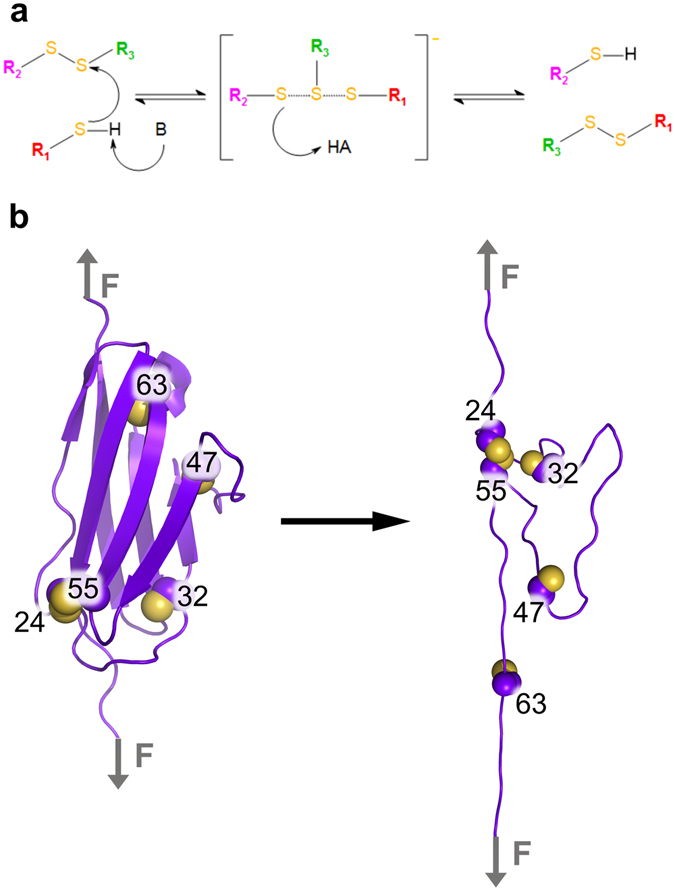
(a) Scheme of acid-base catalyzed thiol-disulfide exchange reaction proceeding through a classical SN2 trigonal bipyramidal transition state. (b) Schematic representation of force-clamp MD simulations during which the I27* domain unfolded under constant force application. The five cysteine residues contained in I27* are highlighted (carbon alpha and carbon beta atoms: purple spheres and sulfur atom: yellow sphere). The disulfide bond between 24Cys and 55Cys prevented the protein from fully stretching and stabilized a closed disorder loop. This loop confined two of the three free cysteines (32Cys and 47Cys) in proximity to the 24Cys–55Cys disulfide bond. The third free cysteine, 63Cys, at the C-terminal part of the protein moved away from the disulfide bond upon stretching.
