Abstract
Studies have highlighted the role of nutritional and metabolic modulators in asthma pathobiology. Steroid resistance is an important clinical problem in asthma but lacks good experimental models. Linoleic acid, a polyunsaturated fatty acid, has been linked to asthma and glucocorticoid sensitivity. Its 12/15–lipoxygenase metabolite, 13-S-hydroxyoctadecadienoic acid (HODE) induces mitochondrial dysfunction, with severe airway obstruction and neutrophilic airway inflammation. Here we show that HODE administration leads to steroid unresponsiveness in an otherwise steroid responsive model of allergic airway inflammation (AAI). HODE treatment to allergic mice further increased airway hyperresponsiveness and goblet metaplasia. Treatment with dexamethasone was associated with increased neutrophilic inflammation in HODE treated allergic mice; unlike control allergic mice that showed resolution of inflammation. HODE induced loss of steroid sensitivity was associated with increased p-NFkB in mice and reduced GR-α transcript levels in cultured human bronchial epithelia. In summary, HODE modifies typical AAI to recapitulate many of the phenotypic features seen in severe steroid unresponsive asthma. We speculate that since HODE is a natural metabolite, it may be relevant to the increased asthma severity and steroid insensitivity in patients who are obese or consume high fat diets. Further characterization of HODE induced steroid insensitivity may clarify the mechanisms.
Introduction
Linoleic acid, a dietary polyunsaturated fatty acid (PUFA), and its lipid metabolites are known to mediate several inflammatory pathways in asthma. Dietary intake of ω-6 and ω-3 fatty acids determines the lipid composition of the cell and its membrane, which in turn affects the cell health. It has been well established that while ω-6 fatty acid and its metabolites are pro-inflammatory, ω-3 fatty acids are majorly anti-inflammatory1. Previous studies have shown a positive association between dietary components rich in ω-6 fatty acid such as margarine and vegetables oils (soy, safflower, sunflower and corn), and asthma prevalence2–8.
Asthma, which was initially thought to be a Th2 dominant disease, is now considered a heterogeneous syndrome with respect to clinical phenotypes and treatment responses9, 10. Among which, obese-asthma (lacks Th2 biomarker) and neutrophil dominant asthma phenotype represent a significant proportion in asthma and respond poorly to corticosteroid treatment11, 12. Although, steroid resistance is seen in only 5–10% of the asthmatic population, it consumes significant health resources and contributes to substantial mortality and morbidity13. Despite being a major hindrance to the treatment, very little is understood of steroid resistance phenotype and its molecular regulators. The lacunae in the understanding of the mechanism could be partially attributed to the lack of good experimental model. Currently available steroid resistant mouse models are induced by using various external triggers or insults such as OVA and House dust mite14–17. However, there are very limited studies with endogenous factors, present upstream of the phenotype observed in steroid resistance. In this context, studying the role of endogenous factors (e.g, lipid metabolites) present in asthmatics may provide better insights into the molecular mechanisms underlying steroid resistance in humans and would be more clinically relevant.
Linoleic acid (ω-6 fatty acid), is known to increase the levels of cytokines which leads to neutrophilia18. These are also known to negatively modulate the binding of synthetic glucocorticoids with glucocorticoid-receptor19, 20. Its 12/15–lipoxygenase metabolite, 13-S-hydroxyoctadecadienoic acid (hereafter written as HODE), is not only increased in asthmatic lungs but also induces mitochondrial dysfunction, severe airway obstruction with neutrophilic inflammation in naïve mice21. Role of dietary lipids and its metabolites is not known in steroid resistance. Hence, in this study, we explored the involvement of HODE in the development of steroid resistance like features of asthma.
Here, we demonstrate for the first time, the importance of a metabolic intermediate, HODE, in the development of steroid resistance. We further provide evidence to support the role of NF-κB and GR-α in the HODE induced steroid insensitivity.
Results
Airway inflammation induced by 13-S-HODE, a dietary lipid metabolite, is resistant to steroid treatment
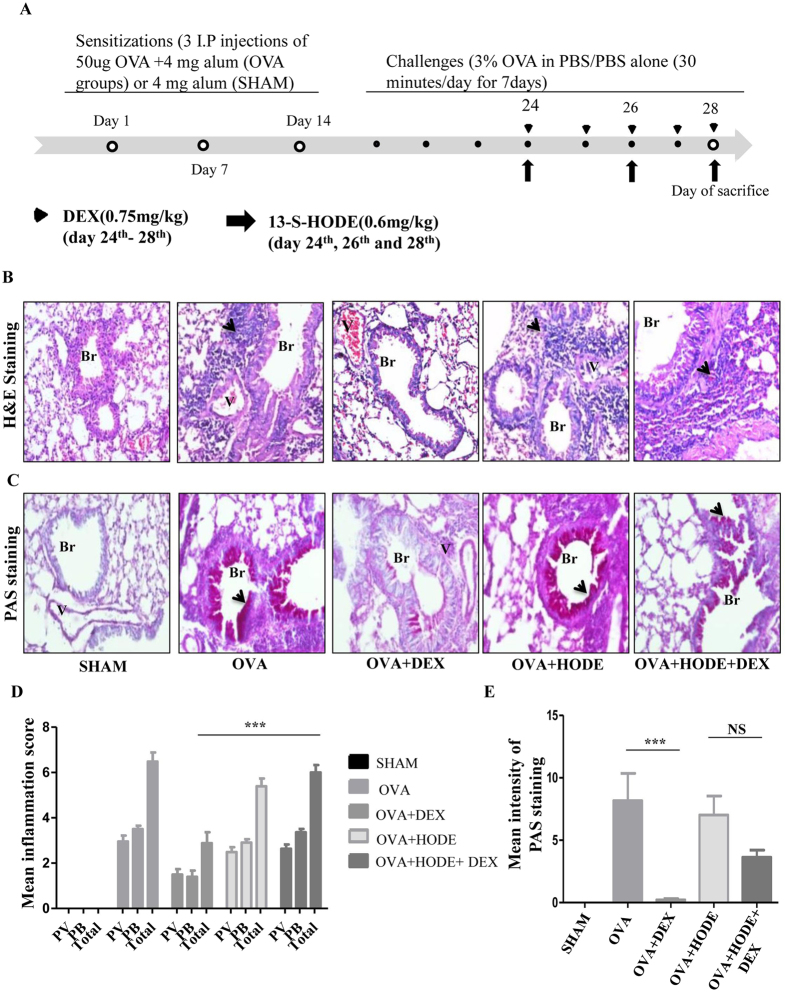
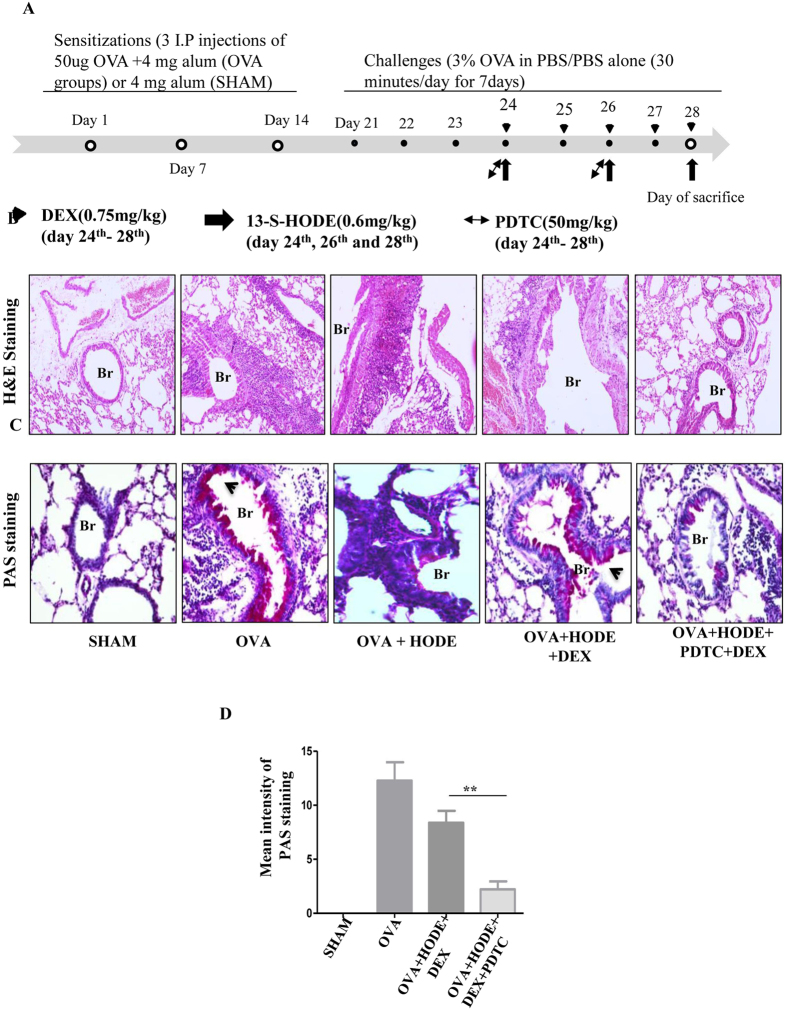
To investigate the effect of HODE on steroid resistance, HODE (0.6 mg/kg or 2.02 mM) was administered to OVA induced allergic mice intranasally (Fig. 1A). As compared to SHAM, OVA induced mice showed increased infiltration of inflammatory cells and goblet cell metaplasia (GCM), which were alleviated with DEX (dexamethasone, a steroid) treatment. However, DEX was unable to reduce inflammatory cell infiltration and GCM in HODE administered allergic mice (OVA + HODE + DEX) (Fig. 1B–E).
Figure 1.
Dexamethasone fails to attenuate airway inflammation and goblet cell metaplasia induced by HODE in allergic mice. (A) Schematic representation of experimental design/protocol as described in methods. (B and C) Representative photomicrographs (20 X magnifications) of bronchovascular regions of different groups of mice stained with haematoxylin and eosin (H & E) and periodic acid–Schiff (PAS). Arrows indicate the infiltrated inflammatory cells in (B) and goblet cell metaplasia in (C). Mean inflammation score (D) and mean intensity of mucin content (E) estimated from the images of H and E and PAS stained lung sections. Data represents mean ± SE; n = 4–6 each group; ***p < 0.001, NS, non-significant (OVA versus OVA + HODE + DEX), Br: bronchi, V: vessel.
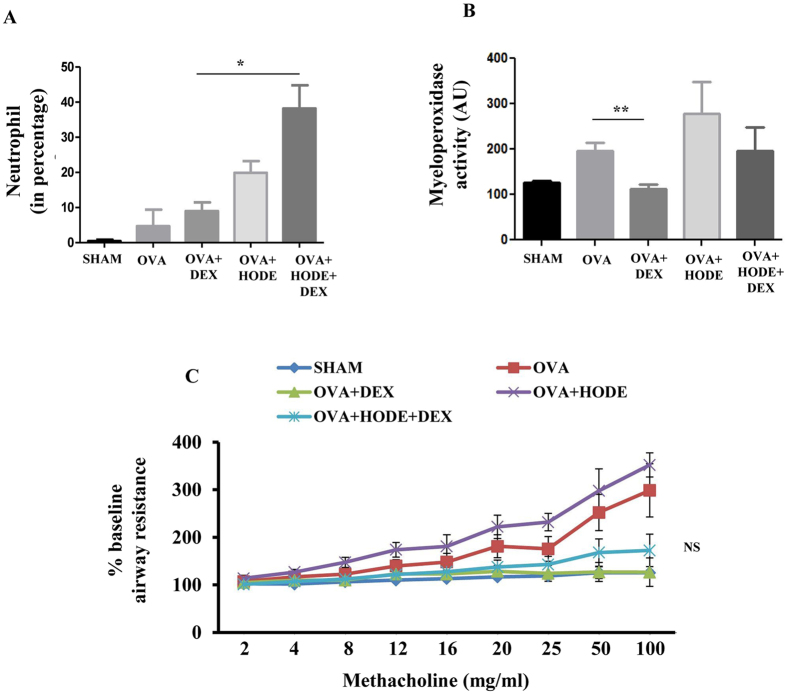
In bronchoalveolar lavage (BAL) fluid, HODE-treated allergic airway inflammation (AAI) mice showed significant increase in eosinophils, lymphocytes and neutrophils. Treatment with DEX was able to reduce the percentage of eosinophils, but not neutrophils (Fig. 2A). In fact, it increased the number of neutrophils in HODE-treated AAI mice. Myeloperoxidase assay suggested that the active neutrophils were significantly reduced by DEX treatment in OVA mice, but not in HODE-treated OVA mice (Fig. 2B). Further, DEX could not reduce airway hyper-responsiveness (AHR) in response to methacholine in OVA mice administered with HODE (OVA + HODE + DEX) when compared to OVA alone mice (OVA + DEX) (Fig. 2C).
Figure 2.
Dexamethasone fails to alleviate HODE induced neutrophilic inflammation and AHR in allergic mice. (A and B) Neutrophil percentage and myeloperoxidase activity in BAL fluid. (C) The percentage baseline airway resistance in response to increasing concentrations of methacholine in HODE administered allergic mice. Data represents mean ± SE; n = 4–6 mice each group; *p < 0.05, **p < 0.01, NS, non-significant (OVA versus OVA + HODE + DEX).
HODE administration reduced GR-α and its activity in human bronchial epithelial cells
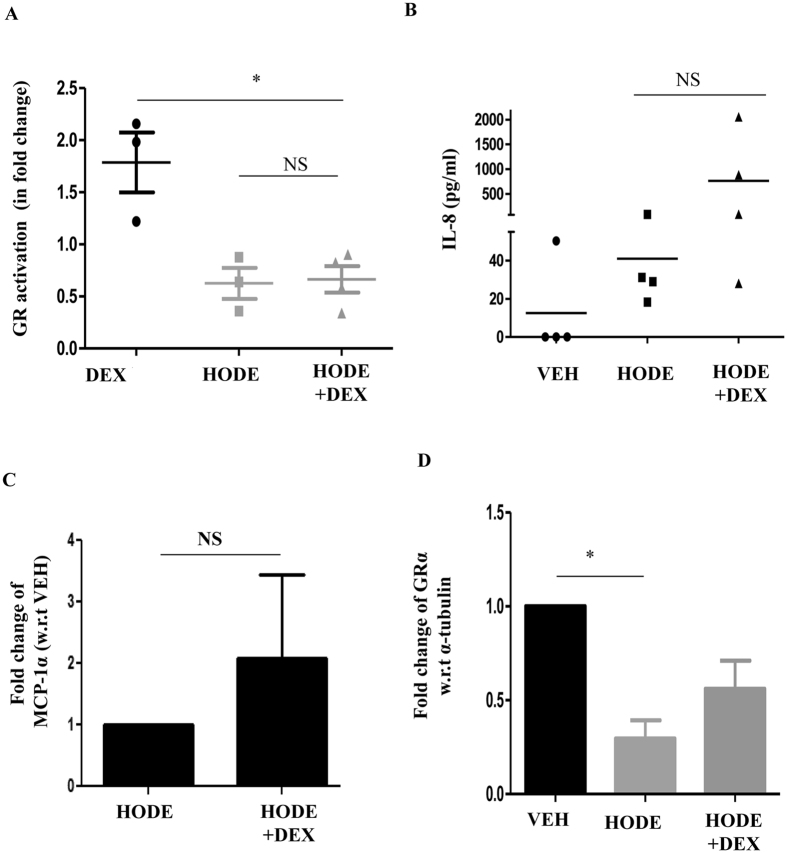
To determine whether HODE-mediated steroid resistance was through direct effects on the glucocorticoid response, we studied the effect of HODE on glucocorticoid receptor. Glucocorticoid response is mediated by glucocorticoid receptor (GR) that binds to glucocorticoid response element (GRE) and modulates the expression of the downstream genes22. To examine whether HODE affects GR activation, binding of GR to synthetic GRE oligonucleotides was estimated in nuclear extracts of dexamethasone pretreated bronchial epithelia (BEAS-2B), which were induced with HODE. HODE reduced the GR activation when compared to dexamethasone alone, and this reduction was not restored with addition of dexamethasone (Fig. 3A). Downstream effects of dexamethasone, such as suppression of IL-8 and MCP-1α, were also abolished by HODE (Fig. 3B,C). To determine whether HODE-mediated reduction in GR activation was due to decrease in the GR-α receptor expression, we measured the transcript levels of GR-α in BEAS-2B cells. HODE treatment led to a significant decrease in the levels of GR-α expression (Fig. 3D).
Figure 3.
HODE reduces GR-α and its activation in BEAS-2B cells. (A) Glucocorticoid receptor (GR) activation was estimated in nuclear extract of HODE induced Beas2B cells (details in methods). (B and C) Levels of IL-8 and MCP1-α in the supernatants of cultured human bronchial epithelia, induced with HODE and DEX. (D) Transcript levels of GR-α normalized to α-tubulin. Data represents mean ± SE; n = 3–5; *p < 0.05, NS: non-significant.
HODE induced inflammation upregulated p-NFκB in allergic mice
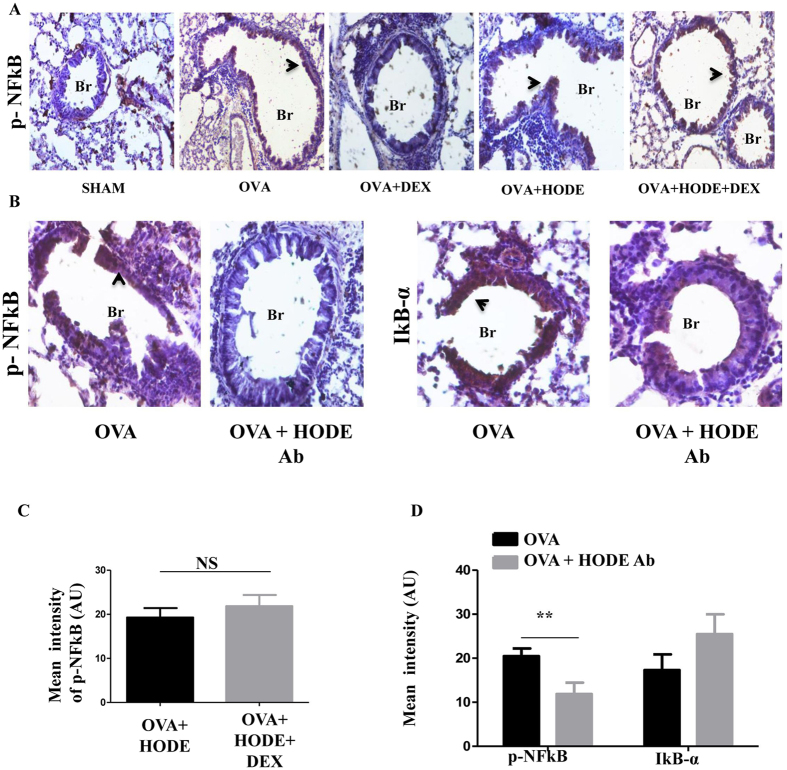
To determine whether HODE-mediated steroid resistance is via the transient receptor potential cation channel subfamily V member 1 (TRPV1), which may mediate HODE-induced asthma like features21, we knocked down TRPV1 in our steroid resistant model. However, siRNA mediated knock down of TRPV1 did not resolve AHR, AAI or MPO activity in this model (Supplementary Fig. 1). Since NF-κB activation is previously reported in steroid resistant asthma23, we next performed immunohistochemical measurement of p-NFκB p65 (Ser 536). We found that OVA challenge led to a DEX-sensitive p-NFκB increase in airway epithelium. HODE treatment was associated with lack of p-NFκB decline post DEX treatment. HODE neutralization was associated with a significant reduction in p-NFκB, along with a trend towards restoration of IκBα levels (Fig. 4A–D).
Figure 4.
HODE administration increases p-NFκB in AAI mice. (A and C) Representative IHC images (20X magnifications) and quantification of the expression of p-NFκB in HODE induced steroid resistance model. (B and D) Representative IHC images (20X magnifications) and quantification of p-NFκB and IκB-α in the lung sections of HODE neutralized allergic mice. Data represents mean ± SE; n = 3–6; **p < 0.01, NS: non-significant. Br: Bronchi. Arrows indicate the positive expression
Inhibition of NF-κB alleviated HODE induced steroid resistant inflammation in allergic mice
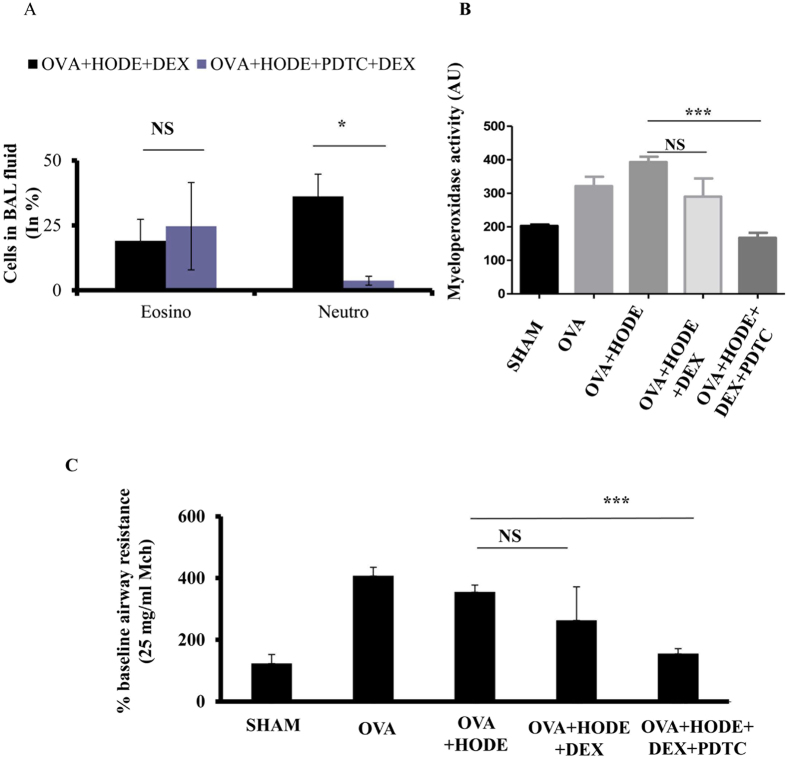
To verify the role of NF-κB in HODE mediated steroid resistance, we tested whether the steroid resistance was reversible by pyrrolidinedithiocarbamate (PDTC, 50 mg/kg), a potent NF-κB inhibitor (Fig. 5A). PDTC increased the sensitivity to DEX in HODE-treated steroid resistant mice. Infiltration of inflammatory cells as well as GCM were reduced in lung sections of PDTC treated OVA + HODE + DEX mice compared to OVA + HODE + DEX mice (Fig. 5B–D). Cells in BAL fluid indicated that PDTC specifically reduced neutrophilic airway inflammation. We also found a significant reduction in myeloperoxidase activity in PDTC treated OVA + HODE + DEX mice, when compared to HODE-treated OVA mice (Fig. 6A,B). PDTC administered mice also showed reduced AHR in response to 25 mg/ml methacholine than HODE-treated OVA mice (Fig. 6C).
Figure 5.
NFκB inhibitor treatment increases steroid sensitivity, reduces airway inflammation and goblet cell metaplasia in HODE induced steroid resistant mice. (A) Schematic representation of experimental design. PDTC (50 mg/kg) was administered intraperitoneally on day 24th and day 26th, 2 hrs and 4 hrs after the administration to HODE and DEX respectively (details explained in material and methods). (B and C) The representative photomicrographs of H and E (20x magnifications) and PAS, respectively. (D) Mean intensity of PAS calculated by Image J software. Data represents mean ± SE; n = 3–6; **p < 0.01, NS: non-significant. Br: bronchi, V: vessel, Arrows indicate the goblet cell metaplasia.
Figure 6.
NFκB inhibitor treatment alleviates neutrophilic inflammation and airway hyperresponsiveness in HODE induced steroid resistant mice. (A) The counts of neutrophils and eosinophils in BAL fluid of steroid resistant mice administered with PDTC. (B) The myeloperoxidase activity in mouse BAL fluid supernatants. (C) The percentage baseline airway resistance in response to 25 mg/ml methacholine dose. Data represents mean ± SE; n = 3–6 each group; *p < 0.05,***p < 0.001, NS: non-significant.
Discussion
While there are numerous reports on the effect of dietary lipids and its metabolites on the pathogenesis and increased incidence of asthma2–8, there are no studies indicating its role in steroid resistant asthma. We, for the first time, show that 13-S-HODE, the 12/15 LOX metabolite of linoleic acid, leads to steroid resistance. This was shown in mice with AAI and in cultured human bronchial epithelial cells. OVA induced AAI mice, which are typically sensitive to steroids, showed steroid resistant airway hyperresponsiveness and goblet cell metaplasia upon HODE induction. In human bronchial epithelial cells, HODE led to decreased GR-α transcript. GR-α mediates the effects of glucocorticoids by further binding to positive or negative glucocorticoid response elements that mediate activation or repression of downstream genes, respectively22. Decrease in the transcript levels of GR-α, is expected to lead to reduced activity of steroids and hence, steroid insensitivity. GR-β, the decoy receptor of GR-α, has also been studied in steroid resistant asthma patients. These studies suggest an increase in the expression of GR-β in the neutrophils24–27. However, in our study, we did not find any significant changes in the expression of GRβ in HODE induced bronchial epithelial cells (data not shown). As for the murine model, the presence of GRβ and its role in steroid resistance remains equivocal. It has been demonstrated using in-vitro cellular systems that unsaturated fatty acids like linoleic acid negatively modulate the binding of triamcinolone acetonide or dexamethasone, a synthetic glucocorticoid, with glucocorticoid-receptor19, 20. With respect to the current study, it would be interesting to investigate the direct or indirect mechanism with which HODE could modulate GR-α.
There are numerous metabolites derived from linoleic acid through its downstream fatty acids like gamma linolenic acid, and arachidonic acid and certain enzymes like Δ6-desaturase, elongase, Δ5-desaturase, lipoxygenases, cyclooxygenase and cytochrome P45028. Many of the linoleic acid metabolites like leukotoxin, isoleukotoxin, leukotrienes are pro-inflammatory mediators29. Though 5-lipoxygenase/leukotrienes pathway are known to be crucial in causing bronchospasm, the role of 15-lipoxygenase and its downstream metabolites were not well explored. In this context, we have shown the involvement of 15-LOX and its metabolites in causing mitochondrial dysfunction in asthma pathogenesis30, 31. Though there are numerous metabolites of linoleic acid,we focused on 13-S-HODE as we found highly increased levels of this in asthmatic patients in our earlier study21. As we have demonstrated the involvement of IL-4/IL-13/15-LOX pathway on mitochondrial dysfunction21, 31, we were interested to see the effects of 13-S-HODE, a downstream metabolite of this pathway on airway function and further steroid resistance. It would be interesting to study the effects of linoleic acid rich diet on the airway function and steroid resistance. These supplementation studies have to demonstrate the levels of linoleic acid and its metabolites in the airway before dissecting the role of linoleic acid diet supplementation on steroid resistance as asthma predominantly affect the airways in general. In this context, it has been shown that linoleic acid supplementation indeed worsens the cystic fibrotic conditions with increased levels of pro-inflammatory mediators like IL-832. So it would be interesting to see the effects of high fat especially linoleic acid diet rich diet on steroid resistance. In any event high fat fed mice had shown the steroid resistant features (Singh VP et al., unpublished data from our lab).
Our group has administered intranasal 13-S-HODE to demonstrate its relation with severe asthma. We indeed calculated the intranasal dosage from the 13-S-HODE levels present in the BAL fluids of human asthmatics in reference to IL-4 and IL-13 levels in BAL fluids of human and mice asthmatic conditions as 13-S-HODE is the product IL-4 and IL-13 signaling. It has been shown in a number of studies that though the BAL fluid concentrations of IL-4 and IL-13 do not differ at basal conditions in asthmatic conditions, it reaches up to 200–400 pg/ml after allergen challenge33–35. To mimic IL-4 or IL-13 mediated human relevant asthmatic condition in mice, 3–5 µg of recombinant IL-4 or recombinant IL-13 per day has been widely used36, 37. So we have used 15 µg of 13-HODE to approximately 25 gram mouse (0.6 mg/kg) as we found approximately 1200 pg/ml of 13-S-HODE in the BAL fluids of human asthmatics21.We did not check the levels of HODE in blood of mice that were administered intranasal HODE and so we are not sure whether intranasal HODE reached bloodstream or not. But there is a good possibility that it might spill over to bloodstream as we administered HODE in the OVA induced inflamed lungs. In addition, we found increased levels of endogenous HODE in sera of human asthmatics21.
Our previous report demonstrates that naïve mice treated with HODE had increased neutrophilia and high Th17 cytokines, which are often seen in patients with steroid resistant asthma12, 13. Though the chemotactic activity of 13-hydroxy-linoleic acid on the isolated exogenous neutrophils is known38, we have shown the in vivo demonstration of HODE induced airway neutrophilia. In the present study, HODE induced steroid resistance in mice with AAI was not associated with any change in IL-17A, IL-21 or IL-22 (data not shown), suggesting that there may be different mechanisms of action of HODE in uninflamed and inflamed lungs. This is supported by our observation that TRPV1 inhibition, which attenuates the effects of HODE in naive mice21, had no effect in the steroid resistant AAI model. Also, it would be interesting to investigate the effects of DEX in HODE administered naïve mice as this could reveal the possible effects of DEX on non-allergic steroid resistant inflammatory conditions with increased levels of 13-S-HODE. However, in human bronchial epithelial cells, DEX treatment in presence of HODE was unable to reduce the steroid responsive cytokines such as IL-8 and MCP-1α. While IL-8 and MCP-1α are steroid regulated cytokines, these cytokines are also known to be regulated by NF-κB. And interestingly, GR-α inhibits NF-kB mediated pro-inflammatory cytokines by physically interacting with p65 subunit (Rel-A), creating a competition for the binding of coactivators and preventing the phosphorylation of RNA polymerase II39. In this scenario, the reduced GR-α in HODE induced BEAS-2B implies the loss of inhibition of GR-α on NF-κB, thereby increasing the expression of cytokines driven by NF-κB. There are substantial reports suggesting the involvement of NF-κB in multiple inflammatory pathways of asthma, some of which also converge with steroid mediated pathways. Similar to these, we also observed that the loss of steroid sensitivity in mice was associated with increased p-NFκB. NF-κB is known to regulate the expression of cytokines such as KC (mouse homologue of IL-8) and G-CSF which helps in neutrophil chemotaxis and survival. We show that pyrrolidinedithiocarbamate (PDTC) administration specifically inhibited the neutrophilic inflammation in HODE induced steroid resistant mice and increased the sensitivity towards steroids, therefore, resolving the steroid resistant features. Although, we clearly observed that HODE induced steroid resistance was due to activated NF-kB, we did not find any significant change in the transcript levels of RelA/p65 in HODE induced BEAS-2B cells (data not shown). Thus, the mechanisms underlying HODE mediated NF-kB activation are yet to be investigated.In any event, the interplay of reduced GR-α and increased p-NFκB thus appears to be critical in the development of HODE-induced steroid resistance (Fig. 7).
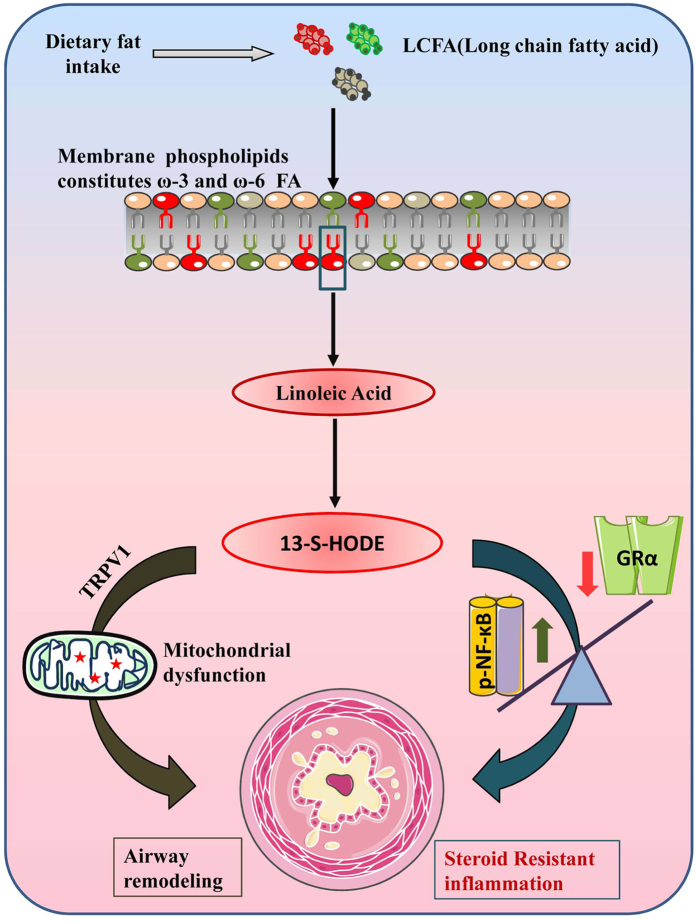
Figure 7.
13-S-HODE mediates steroid resistance via NF-κB and GR-α. Dietary lipids, absorbed through intestine are converted to long chain fatty acids which serve as a precursor for the formation of phospholipids. While, ω-6 fatty acids (red color phospholipid) are oxidized into pro-inflammatory lipid mediators, ω-3 is (green color phospholipid) oxidized to anti-inflammatory. 13-S-HODE is a lipid metabolite of linoleic acid (ω-6 fatty acid), oxidized by 15-lipoxygenase. 13-S-HODE, causes airway remodeling and goblet cell metaplasia by mitochondrial dysfunction via TRPV1 channels. In another independent pathway, it increases p-NFκB and reduces GR-α leading to steroid resistant asthma.
In the present study, we have focused only on the NF-kB pathway. However, the involvement of other pathways relevant to steroid resistance like ERK1/2, JNK, and p38 mitogen-activated protein kinase signaling pathways40 in this steroid resistance model needs to be explored. So, the existence of NF-κB independent mechanisms to regulate the expression of GR has to be investigated in details. However, we envisage the involvement of nuclear receptors regulated by lipid metabolites. Also, it would be interesting to compare the levels of lung HODE among steroid resistant and steroid sensitive patients, although the levels of HODE are known to be increased in sera of asthmatic patients. We speculate that such studies will lead to a greater mechanistic understanding of steroid resistance in asthma and clarify the role of dietary lipids with respect to steroid sensitivity. There is an increase in the dietary ω-6/ω-3 fatty acid ratio due to the westernization of food consumption patterns41. This gradual change in the fatty acid composition of the diet, including an increase in the level of ω-6 fatty acids and ω-6/ω-3 fatty acid ratio with time is associated with increased risk and prevalence of obesity41, which emerges as a risk factor for asthma development42–45. Obese-asthma phenotype requires greater deal of attention and studies as it is typically refractive to steroid treatment43, 45 and this is where we speculate our study can bridge the gap. Moreover, it would be interesting to check the levels of HODE in obese-asthmatic patients and correlate it with the steroid responsiveness to further strengthen the hypothesis. Our study would help in understanding the pathogenesis of steroid resistance, overarching all the phenotypes including obese-asthmatics.
Methods
Mice grouping
The male BALB/c mice (6–8 weeks) were procured from Central Drug Research Institute, Lucknow, India and maintained in Institute of Genomics and Integrative Biology (IGIB), Delhi, India. The Institutional Animal Ethical Committee at IGIB approved all mice experiments and all methods were performed in accordance with the relevant guidelines and regulations of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). Two different allergic models were utilized; first being, OVA model with five groups: SHAM (mice that were PBS sensitized, PBS challenged and treated with vehicle, 50% ethanol), OVA (mice that were OVA, grade V chicken egg ovalbumin, sensitized, OVA challenged and treated with vehicle), OVA + DEX [allergic mice treated with dexamethasone (0.75 mg/kg) orally], OVA + HODE [allergic mice administered with intranasal HODE (0.6 mg/kg or 2.02 mM) and treated with vehicle] and OVA + HODE + DEX [allergic mice administered with intranasal HODE (0.6 mg/kg or 2.02 mM) and treated with dexamethasone (0.75 mg/kg) orally]. The second model had following groups: SHAM, OVA, OVA + DEX, OVA + HODE + DEX, and OVA + HODE + DEX + PDTC [OVA + HODE + DEX mice administered with PDTC dissolved in DMSO (50 mg/kg) intra-peritoneally].
OVA-immunization and challenge
Mice were sensitized with three intraperitoneal injections of 50 µg OVA adsorbed in alum for three weeks and challenged with 3% OVA in PBS for 7 days as described earlier21, 46, 47.
Administration of 13-S-HODE, Dexamethasone, and PDTC
13-S-HODE (Cayman, Michigan,USA) or VEH (50% ethanol) was instilled to the nasal openings of each isoflurane anesthetized mouse. Based on our previous publication21 we have selected the dose of 0.6 mg/kg or 2.02 mM for each mouse. 13-S-HODE was administered intranasally on days 24, 26 and 28 as shown in Fig. 1A. Dexamethasone (Sigma-Aldrich, MO, USA), dissolved in 50% ethanol, was given orally (0.75 mg/kg) to mice from day 24 to 28 as shown in Fig. 1A. PDTC (Sigma-Aldrich, USA) was dissolved in DNAase and RNAase free H2O, and was administered intraperitoneally into mouse (50 mg/kg) on days 24, 26 and 28, 2 hrs after the administration of HODE (Figs 1A and 5A).
Airway hyperresponsiveness measurement and bronchoalveolar lavage (BAL)
Airway hyper-responsiveness was estimated with invasive flexiVent (SCIREQ, Montreal, Canada) as previously described21, 46, 47. BAL was performed and differential cell counts were made as described earlier21, 46, 47.
Lung histopathology
Formalin-fixed lung sections were stained with Haematoxylin & Eosin (H & E), Periodic acid-Schiff and morphometric analysis was performed using publicly available Image J software21, 46, 47.
In vitro experiments
Human bronchial epithelial cells (Beas-2B) were obtained (ATCC, Manassas, VA, USA), maintained in HAM’s F12 (Sigma-Aldrich, MO, USA) with 10% fetal bovine serum (FBS). The cells were pretreated with dexamethasone (10−6 M, Sigma, MO, USA) for 3 hrs before stimulating with vehicle (ethanol) or 13-S-HODE (35 μM, Cayman, Ann Arbor, Michigan, USA) for 16 hrs. These cells were harvested for further experiments and supernatants were stored for cytokine assays.
ELISAs
IL-8, MCP1-α (E-biosciences, CA, USA), myeloperoxidase assay (Cayman chemicals, Michigan, USA) were performed according to manufacturer’s instructions from culture supernatants (IL-8, MCP1-α) and BAL Fluid respectively.
For measuring GR activity (Active Motif, CA, USA) BEAS-2B cells obtained after HODE and DEX treatment (as described above), were processed for nuclear extract. Elisa was performed using manufacturer’s protocol with 20 μg of nuclear extract. The O.D measured is plotted in arbitrary units (AU) in fold change, calculated by O.D test/O.D veh48.
Immunohistochemistry
Immunohistochemical analysis for p-NFkB and IkB-α (Santa Cruz Biotechnology, Texas, USA) was performed with respective secondary antibodies (Sigma, St. Louis, MO, USA).
Real Time PCR
Cells harvested were lysed in RNA lysis solution and RNA was isolated (Qiagen, Germany). cDNA was isolated from RNA using the manufacture’s protocol (ABI, CA, USA). Further real time was performed using kappa FAST Syber green (Roche cycler) using the following primers. Human GR-α, FP: 5′ACGGTCTGAAGAGCCAAGAG3′and RP: 5′CAGCTAACATCTCGGGGAAT3′; Human β-actin, FP: 5′CCAACCGCGAGAAGATGA3′, RP: 5′ CCAGAGGCGTACAGGGATAG3′.
Statistical analysis
All data represents mean ± SEM; n = 3–6 each group; *p < 0.05, **p < 0.01, ***p < 0.001. A p-value more than 0.05 is considered non-significant (NS). Statistical significance of the differences between paired groups was determined with a two-tailed Student’s t test. One-way analysis of variance was used to compare multiple groups by using PRISM software and was evaluated further with a nonparametric Mann-Whitney rank-sum test or Krusker-wallis test wherever appropriate.
Electronic supplementary material
Acknowledgements
This work was supported by the projects BSC 0116, MLP 5502 (CSIR), and GAP 0118 (Department of Biotechnology) and GAP0084 (Department of Science & Technology) at Institute of Genomics & Integrative Biology, Council of Scientific and Industrial Research, Govt. of India. L.P., A.G., and R.R. acknowledge AcSIR (Academy of Scientific and Innovative Research, Coordination Office, Mathura Rd, CRRI, Jasola, New Delhi, Delhi-110020) for their PhD registrations.
Author Contributions
L.P. and U.M. designed and performed experiments, analyzed the results and wrote the manuscript. A.G, R.R., and M.K.Y., performed experiments and analyzed the results. B.S.J., S.V.M., P.A.M., B.G., and A.A. contributed to mice models development, samples acquisition and analyzed the results. All the authors reviewed and approved the final version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-09869-9
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Raphael W, Sordillo LM. Dietary polyunsaturated fatty acids and inflammation: The role of phospholipid biosynthesis. Int. J. Mol. Sci. 2013;14:21167–21188. doi: 10.3390/ijms141021167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arvaniti F, et al. Salty-snack eating, television or video-game viewing, and asthma symptoms among 10- to 12-year-old children: the PANACEA study. J. Am. Diet. Assoc. 2011;111:251–257. doi: 10.1016/j.jada.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 3.Black PN, Sharpe S. Dietary fat and asthma: Is there a connection? Eur. Respir. J. 1997;10:6–12. doi: 10.1183/09031936.97.10010006. [DOI] [PubMed] [Google Scholar]
- 4.Ellwood P, et al. Do fast foods cause asthma, rhinoconjunctivitis and eczema? Global findings from the International Study of Asthma and Allergies in Childhood (ISAAC) phase three. Thorax. 2013;68:351–360. doi: 10.1136/thoraxjnl-2012-202285. [DOI] [PubMed] [Google Scholar]
- 5.Mickleborough TD, Rundell KW. Dietary polyunsaturated fatty acids in asthma- and exercise-induced bronchoconstriction. Eur. J. Clin. Nutr. 2005;59:1335–1346. doi: 10.1038/sj.ejcn.1602250. [DOI] [PubMed] [Google Scholar]
- 6.Spector SL, Surette ME. Diet and asthma: has the role of dietary lipids been overlooked in the management of asthma? Ann. Allergy, Asthma Immunol. 2017;90:371–377. doi: 10.1016/S1081-1206(10)61817-0. [DOI] [PubMed] [Google Scholar]
- 7.Wendell SG, Baffi C, Holguin F. Fatty acids, inflammation, and asthma. J. Allergy Clin. Immunol. 2014;133:1255–1264. doi: 10.1016/j.jaci.2013.12.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wickens K, et al. Fast foods - are they a risk factor for asthma? Allergy. 2005;60:1537–1541. doi: 10.1111/j.1398-9995.2005.00945.x. [DOI] [PubMed] [Google Scholar]
- 9.Holgate, S. T. Pathogenesis of Asthma. Allergy Allerg. Dis. Second Ed. 2, 1608–1631 (2009).
- 10.Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2013;131:636–645. doi: 10.1016/j.jaci.2012.12.1564. [DOI] [PubMed] [Google Scholar]
- 11.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368:804–813. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 12.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat. Med. 2012;18:716–25. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 13.Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2013;131:636–645. doi: 10.1016/j.jaci.2012.12.1564. [DOI] [PubMed] [Google Scholar]
- 14.Duechs, M. J., Tilp, C., Tomsic, C., Gantner, F. & Erb, K. J. Development of a novel severe triple allergen asthma model in mice which is resistant to dexamethasone and partially resistant to TLR7 and TLR9 agonist treatment. PLoS One9 (2014). [DOI] [PMC free article] [PubMed]
- 15.Ito K, et al. Steroid-resistant neutrophilic inflammation in a mouse model of an acute exacerbation of asthma. Am. J. Respir. Cell Mol. Biol. 2008;39:543–550. doi: 10.1165/rcmb.2008-0028OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar RK, Herbert C, Foster PS. Mouse models of acute exacerbations of allergic asthma. Respirology2. 2016;1:842–849. doi: 10.1111/resp.12760. [DOI] [PubMed] [Google Scholar]
- 17.McKinley L, et al. T(H)17 Cells Mediate Steroid-Resistant Airway Inflammation and Airway Hyperresponsiveness in Mice. J. Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimble RF, Tappia PS. Modulation of pro-inflammatory cytokine biology by unsaturated fatty acids. Z. Ernahrungswiss. 1998;37(Suppl 1):57–65. [PubMed] [Google Scholar]
- 19.Lee PC, Struve M. Unsaturated fatty acids inhibit glucocorticoid receptor binding of trout hepatic cytosol. Comp. Biochem. Physiol. B. 1992;102:707–711. doi: 10.1016/0305-0491(92)90067-2. [DOI] [PubMed] [Google Scholar]
- 20.Viscardi RM, Max SR. Unsaturated fatty acid modulation of glucocorticoid receptor binding in L2 cells. Steroids. 1993;58:357–61. doi: 10.1016/0039-128X(93)90038-O. [DOI] [PubMed] [Google Scholar]
- 21.Mabalirajan U, et al. Linoleic acid metabolite drives severe asthma by causing airway epithelial injury. Sci. Rep. 2013;3 doi: 10.1038/srep01349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes PJ. Anti-inflammatory Actions of Glucocorticoids: Molecular Mechanisms. Clin. Sci. 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 23.Schuliga M. NF-kappaB Signaling in Chronic Inflammatory Airway Disease. Biomolecules. 2015;5:1266–1283. doi: 10.3390/biom5031266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinds TD, et al. Discovery of glucocorticoid receptor-beta in mice with a role in metabolism. Mol. Endocrinol. 2010;24:1715–1727. doi: 10.1210/me.2009-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strickland I, et al. High constitutive glucocorticoid receptor beta in human neutrophils enables them to reduce their spontaneous rate of cell death in response to corticosteroids. J Exp Med. 2001;193:585–593. doi: 10.1084/jem.193.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goleva E, et al. Increased glucocorticoid receptor beta alters steroid response in glucocorticoid-insensitive asthma. Am. J. Respir. Crit. Care Med. 2006;173:607–616. doi: 10.1164/rccm.200507-1046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, L. B., Leung, D. Y. M., Martin, R. J. & Goleva, E. Inhibition of histone deacetylase 2 expression by elevated glucocorticoid receptor beta in steroid-resistant asthma. Am. J. Respir. Crit. Care Med.182, 877–883 (2010). [DOI] [PMC free article] [PubMed]
- 28.Giudetti AM, Cagnazzo R. Beneficial effects of n-3 PUFA on chronic airway inflammatory diseases. Prostaglandins Other Lipid Mediat. 2012;99:57–67. doi: 10.1016/j.prostaglandins.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Draper AJ, Hammock BD. Identification of CYP2C9 as a human liver microsomal linoleic acid epoxygenase. Arch. Biochem. Biophys. 2000;376:199–205. doi: 10.1006/abbi.2000.1705. [DOI] [PubMed] [Google Scholar]
- 30.Mabalirajan U, et al. Mitochondrial structural changes and dysfunction are associated with experimental allergic asthma. J. Immunol. 2008;181:3540–3548. doi: 10.4049/jimmunol.181.5.3540. [DOI] [PubMed] [Google Scholar]
- 31.Mabalirajan U, et al. 12/15-lipoxygenase expressed in non-epithelial cells causes airway epithelial injury in asthma. Sci. Rep. 2013;3 doi: 10.1038/srep01540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vij N. Linoleic acid supplement in cystic fibrosis: friend or foe? American journal of physiology. Lung cellular and molecular physiology. 2010;299:L597–8. doi: 10.1152/ajplung.00257.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glaab T, et al. Tidal midexpiratory flow as a measure of airway hyperresponsiveness in allergic mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;280:L565–73. doi: 10.1152/ajplung.2001.280.3.L565. [DOI] [PubMed] [Google Scholar]
- 34.Haczku A, et al. The late asthmatic response is linked with increased surface tension and reduced surfactant protein B in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;283:L755–65. doi: 10.1152/ajplung.00062.2002. [DOI] [PubMed] [Google Scholar]
- 35.Batra V, et al. Bronchoalveolar lavage fluid concentrations of transforming growth factor (TGF)-beta1, TGF-beta2, interleukin (IL)-4 and IL-13 after segmental allergen challenge and their effects on alpha-smooth muscle actin and collagen III synthesis by primary human lu. Clin. Exp. Allergy. 2004;34:437–444. doi: 10.1111/j.1365-2222.2004.01885.x. [DOI] [PubMed] [Google Scholar]
- 36.Wills-Karp M, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 37.Grunig G, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henricks PA, Engels F, van der Vliet H, Nijkamp FP. 9- and 13-hydroxy-linoleic acid possess chemotactic activity for bovine and human polymorphonuclear leukocytes. Prostaglandins. 1991;41:21–27. doi: 10.1016/0090-6980(91)90101-K. [DOI] [PubMed] [Google Scholar]
- 39.Necela BM, Cidlowski J. a. Mechanisms of glucocorticoid receptor action in noninflammatory and inflammatory cells. Proc. Am. Thorac. Soc. 2004;1:239–246. doi: 10.1513/pats.200402-005MS. [DOI] [PubMed] [Google Scholar]
- 40.Bucala R. Approaching the immunophysiology of steroid resistance. Arthritis research & therapy. 2012;14 doi: 10.1186/ar3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simopoulos AP. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients. 2016;8 doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ford ES, Mannino DM, Redd SC, Mokdad AH, Mott JA. Body mass index and asthma incidence among USA adults. Eur. Respir. J. 2004;24:740 LP–744. doi: 10.1183/09031936.04.00088003. [DOI] [PubMed] [Google Scholar]
- 43.Forno E, et al. Decreased response to inhaled steroids in overweight and obese asthmatic children. J. Allergy Clin. Immunol. 2011;127:741–9. doi: 10.1016/j.jaci.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haldar P, et al. Cluster analysis and clinical asthma phenotypes. Am. J. Respir. Crit. Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sutherland ER, Goleva E, Strand M, Beuther DA, Leung DYM. Body mass and glucocorticoid response in asthma. Am. J. Respir. Crit. Care Med. 2008;178:682–687. doi: 10.1164/rccm.200801-076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mabalirajan U, et al. Beneficial effects of high dose of L-arginine on airway hyperresponsiveness and airway inflammation in a murine model of asthma. J. Allergy Clin. Immunol. 2010;125:626–635. doi: 10.1016/j.jaci.2009.10.065. [DOI] [PubMed] [Google Scholar]
- 47.Mabalirajan U, Dinda AK, Sharma SK, Ghosh B. Esculetin restores mitochondrial dysfunction and reduces allergic asthma features in experimental murine model. J. Immunol. 2009;183:2059–2067. doi: 10.4049/jimmunol.0900342. [DOI] [PubMed] [Google Scholar]
- 48.Nader N, Chrousos GP, Kino T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009;23:1572–1583. doi: 10.1096/fj.08-117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.