Abstract
The kynurenine pathway generates multiple tryptophan metabolites called collectively kynurenines and leads to formation of the enzyme cofactor nicotinamide adenine dinucleotide. The first step in this pathway is tryptophan degradation, initiated by the rate‐limiting enzymes indoleamine 2,3‐dioxygenase, or tryptophan 2,3‐dioxygenase, depending on the tissue. The balanced kynurenine metabolism, which has been a subject of multiple studies in last decades, plays an important role in several physiological and pathological conditions such as infections, autoimmunity, neurological disorders, cancer, cataracts, as well as pregnancy. Understanding the regulation of tryptophan depletion provide novel diagnostic and treatment opportunities, however it requires reliable methods for quantification of kynurenines in biological samples with complex composition (body fluids, tissues, or cells). Trace concentrations, interference of sample components, and instability of some tryptophan metabolites need to be addressed using analytical methods. The novel separation approaches and optimized extraction protocols help to overcome difficulties in analyzing kynurenines within the complex tissue material. Recent developments in chromatography coupled with mass spectrometry provide new opportunity for quantification of tryptophan and its degradation products in various biological samples. In this review, we present current accomplishments in the chromatographic methodologies proposed for detection of tryptophan metabolites and provide a guide for choosing the optimal approach.
Keywords: chromatography, kynurenines, kynurenine pathway, tryptophan metabolites, tissue analysis
Abbreviations
- AA
anthranilic acid
- CSF
cerebrospinal fluids
- CMEK
capillary micellar electrokinetic chromatography
- DBD‐F
4‐N,N‐dimethylaminosulfonyl‐7‐nitro‐2,1,3‐benzoxadiazole
- ECNI
electron capture negative ion
- EI
electron impact
- HCV
hepatitis C virus
- HPLC
high pressure liquid chromatography
- ED
electrochemical detection
- FD
fluorescence detection
- IDO
indoleamine 2,3‐dioxygenase
- IFN‐γ
interferon‐gamma
- IL
interleukin
- KAT
kynurenine aminotransferase
- Kyn
kynurenine
- Kyna
kynurenic acid
- NAD+
nicotinamide adenine dinucleotide
- ODS column
octadecyl silica column
- PIC
picolinic acid
- PCA
perchloric acid
- (R)‐DBD‐PyNCS
(R)‐4‐(3‐isothiocyanatopyrrolidin‐1‐yl)‐7‐(N,N‐dimethylaminosulfonyl)‐2,1,3‐benzoxadiazole
- Quin
quinolinic acid
- TCA
trichloric acid
- TDO
tryptophan dioxygenase
- Trp
tryptophan
- XA
xanthurenic acid
- 3HAA
3‐hydroxyanthranilic acid
- 3HKyn
3‐hydroxykynurenine
1. INTRODUCTION
Tryptophan (Trp) is an essential amino acid important for living organism. Less than 1% of dietary Trp is used for protein synthesis, and the rest is degraded through decarboxylation, transamination, hydroxylation, or oxidation 1, leading to generation of physiologically significant compounds such as neuroactive tryptamine, neuroprotective melatonin, or immunosuppressive kynurenine (Kyn). About 80–90% of dietary Trp is metabolized into Kyn by the so‐called kynurenine pathway (Fig. 1) and generation of nicotinamide adenine dinucleotide (NAD), an important enzyme co‐factor. In addition, the methoxyindole pathway utilizes about 1–2% of ʟ‐Trp 2 and provides the neuroactive compounds serotonin and melatonin.
Figure 1.
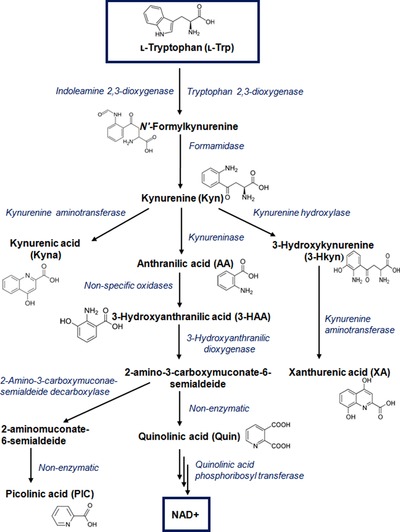
Scheme of ʟ‐tryptophan metabolism via kynurenine pathway
Trp degradation by the kynurenine pathway occurs through several steps and is initiated by activation of two enzymes – extrahepatic indoleamine 2,3‐dioxygenase (IDO; depleting ʟ‐ and ᴅ‐Trp) and hepatic tryptophan 2,3‐dioxygenase (TDO; selective to ʟ ‐Trp) 3, 4. The ratio of kynurenine to tryptophan concentration (Kyn/Trp) reflects IDO and TDO activity and is widely used for monitoring tryptophan metabolism, while the absolute serum Trp level depends on the dietary uptake and is not a reliable parameter 3. Normally, TDO is mainly expressed in the liver and is responsible for regulation of the serum Trp homeostasis. Its expression is induced by corticosteroids 5, however, usually available in liver‐free heme that is necessary for enzymatic activity is sufficient for saturation of only 50% of the available protein.
In contrast IDO is expressed in several mammalian organs, including the endocrine and central nervous systems, placenta, lung, intestine, immune cells, and epididymis 6. The enzyme expression is induced by cytokines, i.e. interferon‐gamma (IFN‐γ), interleukins (IL‐1α, IL‐1β, IL‐6), tumor necrosis factor alpha, and lipopolysaccharide 7, 8, 9, 10 associated with several pathologic conditions such as infection, cancer, as well as with pregnancy 3, 11, 12.
In the first step of kynurenine pathway, ʟ‐Trp is oxidized by IDO or TDO to N′‐formylkynurenine that is rapidly converted to Kyn 13 (Fig. 1). The concentration of this metabolite in plasma, serum, and brain is usually low (micromolar concentrations) 3, 14, 15 and is metabolized by different downstream enzymes within the kynurenine pathway. Kynurenine aminotransferases (KAT I and KAT II) facilitate generation of kynurenic acid (Kyna) by irreversible transamination of Kyn 16, 17. In the brain, Kyna generated by astrocytes 18 occurs at micromolar concentrations, while its level in cerebrospinal fluids (CSF) is much lower (nanomolar concentration) 15. Kyn can however also be a precursor for 3‐hydroxykynurenine (3HKyn) produced by kynurenine hydroxylase, or for anthranilic acid (AA) formed by kynureninase (Fig. 1). Kynurenine hydroxylase is expressed by several cell types including microglia 19, decidual or placental cells 20 and similarly to IDO this enzyme is also upregulated by proinflammatory cytokines 19.
Subsequently, AA is converted to 3‐hydroxyanthranilic acid (3HAA). During 3HAA depletion, the generated 2‐amino‐3‐carboxymuconate‐6‐semialdeide is either metabolized to picolinic acid (PIC) by 2‐amino‐3 carboxymuconate‐semialdehyde decarboxylase 21 or undergoes spontaneous cyclization into Quin (quinolinic acid). This metabolite is required for nicotinic acid (NAD precursor) generation. The concentration of Quin normally present in the nanomolar range in brain and CSF must be kept low, since an increase to 100 nM has been found neurotoxic 22. As a N‐methyl‐d‐aspartate receptor agonist, Quin has been associated with several inflammatory and neurological disorders 23, while PIC shows neuroprotective activity 24.
Since kynurenine pathway is a source of several compounds with diverse biological properties, sometimes causing opposite effects, their concentration is highly regulated in vivo. Disturbance in balance between the kynurenines concentrations may lead to negative effects and often indicates pathology. Trp metabolism has been studied for several decades by many scientists across multiple disciplines. The knowledge obtained brings forth new developments on therapeutic strategies to treat infections, chronic inflammation, cancer, reproduction problems, in addition to useful clinical diagnostics.
Selecting an accurate and reliable method for quantification of individual kynurenines, especially in the complex samples such as tissue, present a challenge in research regarding Trp metabolites. The technological developments, especially recent chromatographic methods coupled with MS detectors, come with novel opportunities however they require costly and sophisticated equipment. Kynurenine detection should also be improved by developing standardized extraction protocols, which require laborious method validation. This review summarizes and analyzes suitability of the existing developments in chromatographic methodologies proposed for quantification of Trp and its kynurenine pathway metabolites. It also brings a handy overview and guide on the selection of an appropriate method for sample preparation and quantification of tryptophan metabolites in variety of biological samples.
2. CLINICAL SIGNIFICANCE OF ABNORMALITIES IN THE KYNURENINE PATHWAY
Products of the kynurenine pathway and IDO regulation are of a special interest in studies on immune activation. Increase in Trp catabolism has been observed in several autoimmune disorders 25. Patients with primary Sjögren's syndrome have higher serum Kyn concentration as well as [Kyn]/[Trp] ratio when compared to control group without autoimmune symptoms 3. Enhanced IDO‐induced Trp degradation has been also observed in systemic lupus erythematosus 8. In addition, multiple other studies have suggested a possible relationship between autoimmune disorders such as encephalomyelitis 18, rheumatoid arthritis 26, 27, and multiple sclerosis 28 with abnormalities of Trp metabolism.
The enhanced IDO expression is also observed in various infections including hepatitis C virus (HCV) 29, 30 where patients with HCV had lower serum Trp level as compared to controls. Furthermore, HCV‐infected subjects frequently suffered from anxiety and depression‐related symptoms, and their macrophages showed low IDO activity 29. On the other hand, PIC appears to have antiviral properties through the reduction of viral replication and increase of apoptosis of the infected cells 31.
Other reports show an implication of Trp and its oxidative pathway products in pregnancy outcome and describe a decreased Trp concentration during normal pregnancy 32 associated with immune activation 32. It is known that IDO is involved in the formation of maternal immune tolerance toward fetus antigens in early pregnancy 20, however the mechanisms ensuring receptivity of the endometrium are not fully understood. The Trp depletion hypothesis involving a reduction of free Trp access from local tissue microenvironment and suppression of T‐cell proliferation 11, 33, 34, 35, 36 is one of the accepted explanation of the Trp and IDO role in immune regulation 37. On the other hand, the Trp utilization concept points to production of downstream Trp metabolites through kynurenine pathway to achieve immune regulation by immunosuppressive kynurenines accumulation and not simple decrease in Trp availability 1, 11, 37. More research on the role of Trp catabolism must be undertaken to explain the mechanism governing normal and abnormal pregnancy, including preeclampsia 38, miscarriage 36, or postpartum blues 39.
Some of psychiatric/neurologic symptoms have been also found to correlate with Trp metabolites produced through kynurenine pathway in brain, CSF, astrocytes, plasma, and serum. The imbalance between neurodegenerative and neuroprotective Trp metabolites might explain the cause of major depression 40 while the increased Trp degradation is involved in neuropathogenesis of Alzheimerʼs disease 41, 42, 43, Huntington's disease 44, Parkinson's disease 45, brain injury 46, 47, AIDS dementia complex 48, meningitis 49, chronic migraine 50, and amyotrophic lateral sclerosis 51. In patients with schizophrenia an enhancement of TDO expression has been demonstrated 52.
The neurodegenerative disorders affecting older people can be explained by the observation that [Kyn]/[Trp] ratio correlates with the level of neopterin, an immune activation marker 53 the concentration of which in the central nervous system increases with age in women 54. The correlating Quin generation leads to neuronal cells death that has relevance to Alzheimer's disease and other dementias 43, 55.
The importance of maintaining the balance between different kynurenines illustrates antagonizing effect of Kyna that blocks neurotoxic Quin 56, 57. An in vitro study has shown that Kyna reduces the dopaminergic neuronal death caused by 1‐methyl‐4‐phenylpyridiniumthe that is the best‐characterized toxin inducing pathology resembling Parkinson's disease 58. Thus, it has been hypothesized that dysfunction of Kyna production may lead to neurologic disorders 56, 59.
The increased Kyna concentration is associated also with other neurological conditions and was found elevated in Alzheimerʼs dementia (in brain) 15 or in schizophrenia (in CSF) 23. Interestingly, the lower concentrations of Kyna have been recorded in CSF of Huntington's disease 57 and depressive patients 48 compared to control individuals. Important contribution to Kyna level has ᴅ‐amino acid oxidase (DAAO, a susceptibility gene of schizophrenia) converting ᴅ‐Kyn to Kyna 59, 60, 61.
The role of kynurenines in mechanism of neurologic disorders was also shown for 3HKyn that is cytotoxic for cultured neuronal cell 62, 63. This metabolite was found to accumulate in patient's brain 64 and is considered a detrimental key player in Alzheimerʼs 41, Huntington's 44 diseases, and hepatic encephalopathy 65.
Attention is drawn by the significant amount of research on possible role of kynurenine pathway in cancer, since Kyn suppresses anti‐tumor immune response leading to development and progression of malignancy. This phenomenon has been mainly attributed to IDO activity 66, 67, 68, 69, however TDO must also be considered in cancer pathogenesis. Optiz and coworkers 67 confirmed that TDO is the central Trp degrading enzyme responsible for Kyn release in human glioma cells. The evidence supporting IDO/TDO overexpression in cancer 70, 71 presents the therapeutic opportunity for tumor rejection induced by agents inhibiting IDO and/or TDO that could have clinical applications 72, 73, 74.
Finally, there are other conditions where Trp metabolites might play a negative role i.e. cataract 75, 76, 77, 78, UV‐induced skin defects 79, and protective role as eye UV filters 80. In addition, there are reports that link kynurenine pathway metabolites with chronic renal insufficiency 81 and suicidal tendency 82.
3. SAMPLE PREPARATION FOR CHROMATOGRAPHIC ANALYSIS OF KYNURENINES
Measurement of Trp and its metabolites is difficult because of their lability, low physiological concentration, and presence of interfering compounds in biological samples. Therefore, some precautions need to be undertaken when performing analysis. Working with samples quickly, at low temperature and protecting from light has been recommended to reduce degradation of Trp and its downstream metabolites to obtain satisfactory results. In case of biological samples such as plasma, serum, or tissues, the protein precipitation before chromatographic analysis must be performed. Proteins in plasma are frequently removed through a pretreatment of sample with acids and by SPE. Deproteinization with perchloric acid (PCA) 14, 44, 61, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101 and trichloroacetic acid (TCA) 29, 54, 101, 102, 103, 104, 105, 106, 107 might be used for this purpose. The sample mixed with acid is shaken and centrifuged to separate precipitated proteins from Trp and kynurenines present in supernatant. However, indole derivatives are sensitive to acidic conditions 99, 108 and precipitation with TCA lowers the Kyn signal 99. Some authors had also proposed the use of other acids like sulfosalicylic acid 95, hydrochloric acid 15, 109, mixture of ascorbic acid, and PCA 26 or TCA with addition of hydrochloric acid 61 or acetonitrile 110. The above agents seem not to be optimal for kynurenines quantification and better choice might be deproteinization with methanol 2, 111, ethanol 112, 113 (chilled or room temperature), and mixture of ammonium acetate in methanol 60, 114, 115, 116 or ammonium acetate in water 117. In some experiments, to prepare brain extracts, acetone has been also used as a precipitation agent 61, 87, 118. Fukushima at al. had reported that extraction efficiency of Kyna with acetone from brain tissue was approximately 1.6‐fold higher than when using 50 mM ammonium acetate in methanol 118. The reprecipitation is also a good practice to purify kynurenines extract 44, 46. Protein precipitation is carried out at room temperature 93, 97, 98, 100, 105 or samples are incubated at −20°C to ensure complete protein removal 2, 111. The subsequent centrifugation is also frequently performed in low temperature (about 4–5°C) to protect indole derivatives from degradation.
Analysis of kynurenines might be performed using samples spiked with internal standard before deproteinization step. It allows for normalization of matrix effects and improves accuracy and reproducibility of the assay 119. There are several compounds used as the internal standards, for determination of Trp and its metabolites, i.e. 3‐nitro‐ʟ‐tyrosie 26, 44, 46, 105, 106, 120, ethyl‐4‐hydroxy‐2‐quinolinecarboxylate 108, theophylline 98, 6‐methyltryptophan 92, ʟ‐tryptophan methyl ester 121, 5‐hydroxytryptamine 122, indoxyl‐sulfate 123, norvaline 40, 7‐aminoheptanoic acid 124, 8‐aminocaprylic acid 125, or dipicolinic acid 46, 126. Many researchers prefer to use the stable isotopically labeled molecules rather than structural analogs especially for bioanalysis employing LC or GC coupled with MS 2, 84, 86, 111, 127, 128, 129, 130. Isotopically‐labeled internal standards have identical chemical properties like the target analyte and minimize problems with stability, recovery, or ionization efficiency issues in comparison to other internal standards. However, the main drawbacks of this approach are high cost and limited availability of the optimal standard 131.
Sample purification by SPE before chromatographic analysis is the useful approach for removing of the interfering compounds present in trace amount. It is based on the nonpolar, polar, ion exchange (cation and anion), and mixed mode interactions of sorbent with an analyte dissolved in liquid phase and subsequent elution with an appropriate solvent. Dowex‐50W cation exchange 15, 84, 109, 132, SepPak 44, 95, and other 41, 102, 106, 108, 116, 120, 129, 133 cartridges have been used for extraction of kynurenines. Implementing of the automated on‐line SPE (using propylsulfonic cartridges) followed by LC–MS/MS shortens the time of analysis reported for quantification of ʟ‐Trp, ʟ‐Kyn, and 3HKyn in human plasma 130. The automated SPE might be also connected with derivatization step of ʟ‐Kyn 115, 116, 120 or Quin to obtain the fluorescent adducts and allow for more sensitive detection 44, 46, 102, 129.
Sample preparation preceding chromatography separation is rather time consuming process, thus methods where sample is directly applied on the column are desired. Kawai's group 134 has described analysis of Kyn and Trp performed by direct injection of filtered plasma (10 μL) into a HPLC system. In this study, the unwanted plasma proteins were removed by trapping on the octadecyl silica precolumn cartridge installed before the main (separation) column 134. This method might generate expected results, but requires frequent replacing of the precolumn due to its heavy wear.
4. CHROMATOGRAPHIC ANALYSIS OF TRYPTOPHAN METABOLITES
4.1. Separation and detection of kynurenines
4.1.1. Determination of free tryptophan
Measurement of Trp concentration in biological samples is mainly performed with simultaneous detection of its degradation products. LC has been often used for this purpose. There are methods employing various detection modalities: UV absorbance 26, 44, 95, 96, 97, 98, 100, 101, 110, 112, 123, 134, 135, fluorescence 14, 29, 40, 54, 92, 93, 94, 104, 105, 106, 110, 133, 136, 137, electrochemical methods 91, 103, 138 as well as MS 2, 86, 99, 111, 120, 121, 124, 128, 130. The LC separation is mostly achieved on octadecyl silica (ODS) columns. The mobile phases usually contain a small addition of acetonitrile (see Table 1) that shortens a retention time and enhances Trp signal 95. HPLC–UV is an attractive method for clinical applications. However, HPLC–UV methods suffer from low selectivity primarily due to the interference of endogenous compounds present in biological samples 96, 103. The simultaneous determination of Trp and its metabolites using HPLC–UV is hampered by other factors, i.e. amphoteric characteristic of Trp, extremely different concentrations in biological samples (e.g. plasma concentrations of Trp and 3HKyn of healthy subjects is about 50 and <0.13 μM, respectively 130) and relatively long time of analysis 124.
Table 1.
Chromatographic protocols for ʟ‐Trp quantification
| HPLC‐UV | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | λ(nm) | Application | Reference |
| 0.069 | 1.22–97.93 | 10% (v/v) CH3CN in H2O, pH adjusted with H3PO4 to 2.7 | 273 |
|
122 |
| 1.32 | 2.45–146.90 | 5 mM CH3COONa, 8% v/v CH3CN | 267 | Human plasma | 112 |
| 0.12 | 3.67–470.00 | 15 mM CH3COONa, 6 % v/v CH3CN, pH adjusted with CH3COOH to 5.5 | 302 | Human plasma | 97 |
| 0.20 | 0.80–500.00 | 15 mM CH3COONa, 5% v/v CH3CN | 225 | Human plasma | 100 |
| 0.05 | 2.25–678.00 | 15 mM acetate buffer (pH 4.0), 5% v/v CH3CN | 278 | Human plasma | 101 |
| 1.18 | 2.00–800.00 | 50 mM phosphate buffer (pH 7.0), 5% v/v CH3CN | 254 | Human plasma | 134 |
| 0.20 | – | 100 mM (CH3COO)2Zn, 50 mM CH3COOH, 3% v/v CH3CN | 250 | Human plasma | 44, 46 |
| 0.02 | − | 100 mM (CH3COO)2Zn, 50 mM CH3COOH, 3% v/v CH3CN | 250 | Human plasma | 26 |
| 1.19 | 3.97–400.00 | 10 mM acetate buffer (pH 4.5), 6% v/v CH3CN | 302 | Human plasma | 98 |
| 1.29 | 5.88–188.00 | A: sodium acetate buffer (pH 4.9)/EtOH/H2O, B: 100% v/v CH3CN, C: 100% v/v H2O, D: 1% v/v sodium acetate buffer (pH 5.85) | 250 | Human plasma | 112 |
| 0.20 | 4.90–490.00 | 15 mM CH3COONa, 2.7% v/v CH3CN (pH 3.6) | 225 | Human serum | 96 |
| 3.50 | 3.51–225.00 | A: 0.1% v/v TCA in H2O, B: 0.1% v/v TCA in MeOH | 280 | Rat serum | 110 |
| − | up to 490.00 | 40 mM acetate/citrate buffer (pH 4.5), 2.5% v/v CH3CN | 254 | Human urine | 123 |
| − | − | 40 mM CH3COONa/citric acid buffer (pH 5), 5% v/v CH3CN | 280 | Dendritic cells | 107 |
| HPLC‐FD | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | λex/λem (nm) | Application | Reference |
| 0.40 | 10.00–100.00 | 20 mM CH3COONa, 3 mM (CH3COO)2Zn, 7% v/v CH3CN | 344/398 | Human plasma | 14 |
| − | − | A: Na2HPO4 in CH3CN (pH 6.5), B: 42% v/v H2O, 28% v/v CH3CN, 32% v/v MeOH | 340/440 | Human plasma | 40 |
| − | 0.90 – 26.00 | 15 mM acetate buffer (pH 4.0), 27 mM CH3CN | 286/366 | Human serum | 105 |
| − | 0.06 – 222.00 | 15 mM potassium phosphate buffer (pH 6.4), 2.7 % v/v CH3CN | 285/365 | Human serum | 106 |
| 0.03 | 0 – 1000.00 | 50 mM CH3COOH, 250 mM (CH3COO)2Zn (pH 4.9), 1% v/v CH3CN | 254/404 | Human serum | 94 |
| 0.001 | 0.49. – 196.00 | 50 mM CH3COONa, 500 mM (CH3COO)2Zn, 6% v/v CH3CN | 254/404 | Human serum | 93 |
| 0.70 | 6.25 – 100.00 | 5 mM (CH3COO)2Zn, 8% (v/v) CH3CN, pH adjusted to 4.9 | 254/404 | Human serum | 92 |
| 0.16 | 1.00 – 50.00 | 15 mM phosphate buffer (pH 4.51) | 254/404 | Human serum | 113 |
| 0.02 | 0.05 – 1000.00 | 30 mM phosphate buffer (pH 8.0), 25% v/v MeOH, tetrabutylammonium hydrogen sulfate | 285/360 | Human serum | 83 |
| − | − | A: 50 mM CH3COONa (pH 4.8), B: 50 mM CH3COONa (pH 3.65), C: 100% v/v CH3CN, D: 100% v/v MeOH | 360/350 | Human serum | 41 |
| 0.005 | 0.05 – 49.00 | 10% v/v CH3CN in H2O | 285/353 | Human serum | 137 |
| − | − | 100 mM phosphate buffer (pH 3.6), 1 mM EDTA, 5% v/v CH3CN | 313/420 |
|
30 |
| − | − | 40 mM CH3COONa/citric acid buffer (pH 5), 5% v/v CH3CN | 286/366 | Dendritic cells | 107 |
| 0.75 | 2.50–100.00 | A: 15 mM phosphate buffer (pH 6.4), B: 100% v/v CH3CN | 254/404 | Amniotic fluids | 136 |
| − | − | 0.1 M CH3COONH4 (pH 4.65) | 254/404 | Human CFS | 54 |
| 1.30 | − | A: 20 mM CH3COONH4 in H2O (pH adjusted to 5.0 with CH3COOH), B: 100% v/v CH3CN | 285/365 | Rat plasma | 62 |
| 0.005 | − | 90% v/v H2O, 10% v/v CH3CN, 0.1% TCA | 297/348 |
|
152 |
| HPLC‐ED | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | Application | Reference | |
| 0.40 | 0.80–160.00 | 0.1 M CH3COONa, 0.1 M citric acid, 27 μL EDTA, 20% v/v MeOH, pH adjusted to 5.0 with 5 M NaOH | Human plasma | 103 | |
| 0.24 | 0.30–100.00 | 94% v/v 16.2 mM KH2PO4, 6% v/v CH3CN |
|
138 | |
| 0.009 | 0.003–73.40 | 50 mM sodium phosphate‐acetate buffer (pH 4.1), 10% v/v MeOH, 0.42 mM octanesulphonic acid | Rat brain | 91 | |
| CMEKC‐ED | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | Application | Reference | |
| 0.004 | 0.03 – 10.00 | 10 M Na2HPO4‐NaOH buffer (pH 11.0), 40 mM sodium dodecyl sulfate, 3% v/v MeOH | Rabbit urine | 143 | |
| LC‐MS or LC‐MS/MS | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | Monitored ions / transitions (m/z) | Application | Reference |
| 0.003 | 0.006 – 95.00 | A: 2.1% v/v HCOOH (pH 2.0), B: 2.1% v/v HCOOH, 40% v/v CH3CN, C: 2.1% v/v HCOOH, 90% v/v CH3CN | 206>189 | Human plasma | 99 |
| 0.25 | 6.12–97.93 | 2% v/v CH3CN, 5.2% v/v MeOH, 0.1% HCOOH | 204.9>187.9 | Human plasma | 128 |
| 0.40 | 0.50 – 400.00 | A: 650 mM CH3COOH, B: 100 mM heptafluorobutyric acid, C: 90% v/v CH3CN in H2O | 206.3>189.1 | Human plasma | 86 |
| 0.03 | 0.11 – 1200.00 | A: 0.2% v/v HCOOH, B: 100% v/v CH3CN | 205>188>146>118 | Human plasma | 130 |
| 0.018 | 0.07–1.126 | A: HCOONH4 in H2O (0.05% v/v), pH adjusted to 5.5 with CH3COOH, B: 100% v/v CH3CN | 204.9>188.1>117.8 | Rat plasma | 108 |
| 0.00005 | 50.00–200.00 | 10% v/v 10 mM HCO2NH4 (pH 5.0) in H2O, 90% v/v CH3CN | 556.15>203.25 | Human serum | 120 |
| 0.15 | 10.00–200.00 | A: 80% v/v H2O, 20% v/v CH3CN, 0.1% v/v CH3COOH, B: 20% v/v H2O, 80% v/v CH3CN, 0.1% v/v CH3COOH | 556.14 | Human serum | 124 |
|
|
A: 0.1% v/v CH3COOH, B: 100% v/v MeOH | 205>146>188 |
|
2 |
| 0.0015 | 0.0015 – 2.45 | A: 0.1% v/v HCOOH in H2O, B: 0.1% v/v HCOOH in H2O | 427 | Human urine | 140 |
| 0.0036 | − | A: 28% v/v 0.4 M CH3COONH4, 32% v/v MeOH, 40% v/v CH3CN, B: 32% v/v MeOH, 68% v/v CH3CN | 383>387>389 | Cell culture medium | 127 |
| 0.01 | 0.01 – 10.00 | A: 5 mM HCOONH4, 0.01% v/v TCA, B: 100% v/v MeOH | 205>146 |
|
121 |
| − | − | A: 0.1% v/v HCOOH in H2O, B: 0.1% v/v HCOOH in CH3CN | 205.1>118.1 | Rat hepatocytes | 110 |
| 0.01 | 0.05 – 200.00 | A: 0.1% v/v HCOOH in H2O, B: 0.1% v/v HCOOH in CH3CN | 205.1>118.1 |
|
111 |
| 0.049 | 0.049 – 9.79 | A: 20 nM CH3COONH4, 0.1% v/v HCOOH in H2O, B: 100% v/v CH3CN | 439>170 | Rat brain | 141 |
| GC‐MS or GC‐MS/MS | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Monitored ions/ transitions (m/z) | Application | Reference | |
| 0.00042 | − | 383 > 387 > 389 | Cell culture medium | 127 | |
| − | 0.01 – 1.00 | 608 > 351 | Rat brain | 119 | |
| − | 2.50 – 80.00 | 608 |
|
102 | |
LOD, limit of detection; CR, calibration range; λ, wavelength; λex, excitation wavelength; λem,‐ emission wavelength.
LC–ESI‐MS/MS (LC–ESI‐MS) has been proposed to determine ʟ‐Trp and its predominant metabolites of the kynurenine pathway 2, 86, 108, 111, 121, 128. The MS/MS, i.e. LC–MS/MS allows for monitoring many kynurenines and other biologically active compounds such as amino acids, vitamins in single experiment. The small amount of the sample, a short time, and high separation efficiency are the advantages of this method. There are several examples of successful application of LC–MS in analysis of kynurenines, i.e. Möller at al. reported a LC–MS/MS method for simultaneous detection of six different kynurenines (ʟ‐Trp and ʟ‐Kyn, Kyna, AA, 3HAA, Quin) in 10 μL of rat plasma. The target analytes were separated within 10 min analysis 108. Midttun and coworkers demonstrated applicability of LC–MS/MS for determination of 16 compounds including ʟ‐Trp, ʟ‐Kyn, Kyna, 3HKyn, 3HAA, XA (xanthurenic acid), and AA and 13 isotope‐labeled internal standards in human plasma 86. Hényková at al. have proposed a UHPLC–MS/MS protocol for quantitative profiling of ʟ‐Trp, ʟ‐Kyn, Kyna, 3Hkyn, 3HAA, AA, and 11 other tryptophan‐related neuroactive compounds 2 in human serum and CSF within 10 min. Despite impressive developments the main drawback of the LC–MS approaches is a low ionization response of Trp and kynurenines compared to less polar compounds (probably due to their lower surface activity during the electrospray droplet formation), and a significant impact of sample impurities on ionization process 108, 139. Thus, the LC–MS methods require careful sample preparation (i.e. by SPE) that will reduce matrix interference and improve extraction efficiency 108, 140. The addition of internal standards (especially isotope‐labeled analogues) minimizes assay variation. The derivatization step might help in analysis to improve sensitivity and specificity of the method since it increases mass of the target compounds eliminating the interference of matrix components occurring in the low‐m/z region 141.
In comparison to LC–MS/MS approaches, the HPLC methods can be also used for simultaneous quantification of a large number of analytes, like it has been done to determine 33 different compounds including ʟ‐Trp and ʟ‐Kyn in human plasma 112. This protocol 112 includes the complex gradient program with four with solvents supplied from 4 different reservoirs, derivatization of analytes and employs two detectors (fluorescence and UV).
The LC separation of target analytes is mostly achieved on octadecyl silica (ODS) columns, however in some reports a triazole‐bonded column has been used. The authors improved sensitivity and specificity of LC–MS/MS assay while working on determination of ʟ‐Trp and ʟ‐Kyn in human serum 120. The method allows ʟ‐Trp and ʟ‐Kyn detection at 50 and 4 pM, respectively. This approach requires derivatization of ʟ‐Trp and ʟ‐Kyn with (R)‐4‐(3‐isothiocyanatopyrrolidin‐1‐yl)‐7‐(N,N‐dimethylaminosulfonyl)‐2,1,3‐benzoxadiazole, (R)‐DBD‐PyNCS, and purification using SPE. In contrast, an LC–MS protocol employing an ODS column for ʟ‐Trp and ʟ‐Kyn determination in human serum using precolumn derivatization with (R)‐DBD‐PyNCS does not require laborious clean‐up step but it compromises the detection limits of ʟ‐Trp and ʟ‐Kyn to 150 nM 124. The derivatization with dansyl chloride for quantification of ʟ‐Trp and other kynurenines simultaneously with other neuroactive metabolites of dopamine and serotonin metabolic pathway has been also proposed for LC–MS/MS 140, 141. Dansylation and introduction of tertiary amine makes the target compounds easily protonated in positive ESI mode by reducing their polarity. This in consequence increases retention on the reversed‐phase column allowing for better separation of compounds 140.
There are also other chromatographic techniques utilized for determination of ʟ‐Trp (see Table 1). They include capillary GC with negative ion MS 142 or GC with electron capture negative ion MS 119, 127, and capillary micellar electrokinetic capillary chromatography (CMEK) with amperometric detection 143. CMEK is an electrophoretic technique, where the separation is based on the differential migration of the ionic micelles and the bulk running buffer, allowing for higher selectivity.
4.1.2. Kynurenine determination
The most popular method of ʟ‐kynurenine (ʟ‐Kyn) quantification is HPLC with UV detection 14, 26, 29, 40, 41, 44, 54, 82, 90, 92, 94, 96, 97, 98, 101, 105, 106, 112, 113, 123, 134, 135, 136. However, sensitivity 89 and selectivity 96, 103 using HPLC‐based methods may be insufficient for determination of ʟ‐Kyn present at micromolar concentrations in biological fluids or tissues. ʟ‐Kyn quantification using UV detector is performed usually at wavelength of 225 and 360 ± 5 nm (see Table 2). Due to high impact of endogenous compounds observed at 225 nm the favored in clinical analysis is detection at 360 ± 5 nm 41, 92, 98. To improve sensitivity, the fluorescence detectors coupled with HPLC (HPLC–FD) are used for ʟ‐Kyn analysis. This method requires ʟ‐Kyn derivatization and generation of the fluorescent ʟ‐Kyn adduct utilizing the precolumn 115, on‐column 89 or postcolumn 144 methods. The precolumn derivatization of ʟ‐Kyn can be carried out using a benzofurazan‐type reagent: 4‐N,N‐dimethylaminosulfonyl‐7‐nitro‐2,1,3‐benzoxadiazole (DBD‐F) 115 and generation of the fluorescent adduct. The postcolumn derivatization method developed by Mawatari's group requires a photochemical reaction of ʟ‐Kyn with hydrogen peroxide 144. While the precolumn fluorescence derivatization approach is more complex and time‐consuming, the postcolumn ones require a complicated equipment. Alternatively, the on‐column derivatization techniques seem to be a good choice for a rapid and sensitive ʟ‐Kyn determination, i.e. Lou at al. employed the on‐column fluorescent derivatization of ʟ‐Kyn by zinc acetate 88. This method greatly enhances a fluorescence response of Kyna (see next subsection) and was applied for ʟ‐Kyn determination in human serum. The on‐column derivatization with zinc acetate has been also used for measurement of ʟ‐Kyn and Kyna levels in human serum samples in a single analytical run 89.
Table 2.
Chromatographic methods for ʟ‐Kyn determination
| HPLC‐UV | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR(μM) | Mobile phase composition | λ(nm) | Application | Reference |
| 0.03 | 1.00 – 10.00 | 20 mM CH3COONa, 3 mM (CH3COO)2Zn, 7% v/v CH3CN | 365 | Human plasma | 14 |
| 0.02 | 0.08 – 50.00 | 15 mM sodium acetate‐acetic acid, 5% v/v CH3CN | 225 | Human plasma | 61 |
| 0.014 | 0.44 – 18.30 | 15 mM sodium acetate buffer,6 % v/v CH3CN, pH adjusted with CH3COOH to 5.5 | 360 | Human plasma | 97 |
| 0.03 | 0.20 – 21.20 | 15 mM acetate buffer (pH 4.0), 5 % v/v CH3CN | 360 | Human plasma | 101 |
| 0.74 | 1.50 – 600.00 | 50 mM phosphate buffer (pH 7.0), 5% v/v CH3CN | 254 | Human plasma | 134 |
| 0.05 | − | 100 mM (CH3COO)2Zn, 50 mM CH3COOH, 3% v/v CH3CN | 365 | Human plasma | 44, 46 |
| – | − | 250 mM (CH3COO)2Zn, 0.9% v/v CH3CN, pH adjusted to 5.8 with CH3COOH | 365 | Human plasma | 40 |
| 0.13 | 0.42– 20.20 | 10 mM acetate buffer (pH 4.5), 6% v/v CH3CN | 302 | Human plasma | 98 |
| 0.05 | − | 100 mM (CH3COO)2Zn, 50 mM CH3COOH, 3% v/v CH3CN | 365 | Human plasma | 26 |
| 0.61 | 1.84– 39.96 | A: sodium acetate buffer (pH 4.9)/EtOH /H2O, B: 100% CH3CN C: 100% H2O, D: 1% v/v sodium acetate buffer (pH 5.85) | 250 | Human plasma | 112 |
| − | − | 0.1 M CH3COOH, 0.1 M CH3COONH4 (pH 4.65), 2% v/v CH3CN | 365 | Rat plasma | 81 |
| − | 0.06 – 1.71 | 15 mM acetic acid‐sodium acetate (pH 4.0), 27 mM CH3CN | 360 | Human serum | 105 |
| − | 0.09 – 9.84 | 15 mM potassium phosphate buffer (pH 6.4), 2.7% v/v CH3CN | 360 | Human serum | 106 |
| 2.00 | 0.06 – 6.25 | 5 mM (CH3COO)2Zn, 8% v/v CH3CN (pH adjusted to 4.9) | 365 | Human serum | 92 |
| 0.02 | 0.098 – 49.00 | 15 mM sodium acetate‐acetic acid, 2.7% v/v CH3CN (pH 3.6) | 225 | Human serum | |
| 0.70 | 0.25 – 10.00 | 15 mM phosphate buffer (pH 4.51) | 230 | Human serum | 113 |
|
0.00 – 100.00 | 50 mM CH3COOH, 250 mM (CH3COO)2Zn (pH 4.9), 1% v/v CH3CN |
|
Human serum | 94 |
|
0.09 – 4000.00 | 30 mM phosphate buffer (pH 8.0), 25% v/v MeOH, tetrabutylammonium hydrogen sulfate |
|
Human serum | 83 |
| − | − | A: 50 mM CH3COONa (pH 4.8), B: 50 mM CH3COONa (pH 3.65), C: 100% v/v CH3CN, D: 100% v/v MeOH | 365 | Human serum | 41 |
| − | − | 0.1 mM CH3COONH4, 0.1 M CH3COOH, 2% v/v CH3CN | 365 |
|
12 |
| 1.50 | 0.43 – 28.00 | A: 0.1% v/v TCA in H2O, B: 0.1% v/v TCA in MeOH | 360 | Rat serum | 110 |
| − | − | 40 mM CH3COONa/citric acid buffer (pH 5), 5% v/v CH3CN | 360 | Dendritic cells | 2 |
| − | up to 480.50 | 40 mM acetate‐citrate buffer (pH 4.5), 2.5% v/v CH3CN | 254 | Human urine | 123 |
| − | − | 0.1 M CH3COONH4 (pH 4.65) | 365 | Human CFS | 54 |
| 0.08 | 0.25 – 50.00 | A: 15 mM phosphate buffer (pH 6.4), B: 100% v/v CH3CN | 230 | Amniotic fluids | 136 |
| HPLC‐FD | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | λex/λem(nm) | Application | Reference |
| 0.04 | 0.10 – 98.00 | 250 mM (CH3COO)2Zn, 50 mM CH3COOH, 3% v/v CH3CN | 365/480 | Human serum | 88 |
| 0.05 | 0.10 – 19.60 | 250 mM (CH3COO)2Zn, 3% v/v CH3CN | 365/480 | Human serum | 89 |
| 0.50 | 0.50 – 50.00 | A: 95% v/v H2O, 5% v/v MeOH, 0.1% HCOOH, B: CH3CN/MeOH (95/5), 0.1% HCOOH | 553/431 | Rat plasma | 115 |
| 0.02 | − | 97% v/v 0.05 M Na2B4O7 0.1 M KH2PO4 buffer (pH 8.5), 3% v/v EtOH, 60 mM H2O2 | − | Human plasma | 144 |
| − | − | 250 mM (CH3COO)2Zn, 50 mM CH3COONa, 3% v/v CH3CN, pH adjusted to 6.2 with CH3COH | 365/480 |
|
61 |
| 0.01 | − | 90% v/v H2O, 10% v/v CH3CN, 0.1% TCA | 364/480 |
|
152 |
| HPLC‐ED | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | Application | Reference | |
| 0.50 | 1.00–80.00 | 0.1 M CH3COONa, 0.1 M citric acid, 27 μL EDTA, 20% v/v MeOH, pH adjusted to 5.0 with 5 M NaOH | Human plasma | 103 | |
| 0.06 | 0.07–10.00 | 94% v/v 16.2 mM KH2PO4, 6% v/v CH3CN |
|
133 | |
| 0.0062 | 0.003–72.04 | 50 mM sodium phosphate‐acetate buffer (pH 4.1), 10% v/v MeOH, 0.42 mM octanesulphonic acid | Rat brain | 94 | |
| CMEKC‐ED | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | Application | Reference | |
| 0.0035 | 0.08–10.00 | 10 M Na2HPO4‐NaOH buffer (pH 11.0), 40 mM sodium dodecyl sulfate, 3% v/v MeOH | Rabbit urine | 143 | |
| LC‐MS or LC‐MS/MS | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | Monitored ions / transitions (m/z) | Application | Reference |
| 0.0034 | 0.006–95.00 | A: 2.1% v/v HCOOH (pH 2.0), B: 2.1% v/v HCOOH, 40% v/v CH3CN, C: 2.1% v/v HCOOH, 90% v/v CH3CN | 209>192 | Human plasma | 99 |
| 0.024 | 0.30–4.80 | 2% v/v CH3CN, 5.2% v/v MeOH, 0.1% v/v HCOOH | 209>192.1 | Human plasma | 128 |
| 0.007 | 0.0005–4.00 | A: 650 mM CH3COOH, B: 100 mM heptafluorobutyric acid, C: 90% v/v CH3CN in H2O | 209.1>174.3 | Human plasma | 86 |
| 0.001 | 0.05–45.01 | A: 0.2% v/v HCOOH, B: 100% v/v CH3CN | 209>94>192>146 | Human plasma | 130 |
| 0.017 | 0.07–1.11 | A: HCOONH4 in H2O (0.05% v/v), pH adjusted to 5.5 with CH3COOH, B: 100 % v/v CH3CN | 208.97>192>93.8 | Rat plasma | 108 |
| 0.20 | − | A: 20 mM CH3COONH4 in H2O, pH adjust to 5.0 with CH3COOH, B: 100% v/v CH3CN | 209.1 | Rat plasma | 62 |
| 0.00004 | 1.00 – 5.00 | 10% v/v 10 mM HCO2NH4 (pH 5.0) in H2O, 90% v/v CH3CN | 560.15>217.20 | Human serum | 120 |
| 0.15 | 0.50 – 5.00 | A: 80% v/v H2O, 20% v/v CH3CN, 0.1% v/v CH3COOH, B: 20% v/v H2O, 80% v/v CH3CN, 0.1% v/v CH3COOH | 560.13 | Human serum | 124 |
|
|
A: 0.1% v/v CH3COOH, B: 100% MeOH | 209>94>192 |
|
2 |
| 0.00002 | 0.0004 – 2.40 | A: 0.1% v/v HCOOH in H2O, B: 0.1% v/v HCOOH in H2O | 442 | Human urine | 140 |
| 0.02 | 0.02 – 10.00 | A: 5 mM HCOONH4, 0.01% v/v TCA, B: 100% MeOH | 209>146 |
|
121 |
| − | − | A: 0.1% v/v HCOOH in H2O, B: 0.1% v/v HCOOH in CH3CN | 209.1>192 | Rat hepatocytes | 110 |
| 0.048 | 0.05–9.61.00 | A: 20 nM CH3COONH4, 0.1% v/v HCOOH in H2O, B: 100% v/v CH3CN | 442>170 | Rat brain | 101 |
| 0.00004 | 0.001–7.50 | A: 0.1% v/v HCOOH in H2O, B: 0.1% v/v HCOOH in CH3CN | 209.1>192.0 |
|
111 |
| 0.017 | − | A: 28% v/v 0.4 M CH3COONH4, 32% v/v MeOH, 40% v/v CH3CN, B: 32% v/v, MeOH, 68% v/v CH3CN | 383>387>389 | Cell culture medium | 127 |
| GC‐MS or GC‐MS/MS | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Monitored ions/ transitions (m/z) | Application | Reference | |
| 0.00055 | − | 387>391/393>393 | Cell culture medium | 127 | |
| − | 0.01 – 1.00 | 612>442 | Rat brain | 119 | |
| − | 1.25 – 40.00 | 454 |
|
102 | |
LOD, limit of detection; CR, calibration range; λ, wavelength; λex, excitation wavelength; λem, emission wavelength.
The LC–MS methods 104, 124 or LC–MS/MS 2, 86, 108, 111, 120, 121, 128, 141 have also been proposed for estimation ʟ‐Kyn levels in biological samples simultaneously with other Trp metabolites. Several of the developed methods for ʟ‐Kyn determination using different chromatographic approaches are compared in Table 2.
Recent studies include exploration and comparison of physiological functions of different enantiomers of Trp and Kyn. The individual or simultaneous ʟ,ᴅ‐Trp and ʟ,ᴅ‐Kyn determination requires a method to separate of chiral compounds. The methods employing precolumn diastereomer derivatization followed by HPLC separation of Kyn and/or Trp enantiomers and their simultaneous fluorescence detection 116, 125, 145 is used for this purpose. Determination of ᴅ‐Kyn in tissues can be also performed with HPLC–FD. This method employs enzymatic oxidation of ᴅ‐Kyn by ᴅ‐amino acid oxidase and subsequent fluorescent detection of the generated Kyna 61, 112, 146, 147.
4.1.3. Determination of kynurenic acid
The most often employed method for Kyna estimation in biological samples such as plasma 14, 26, 40, 87, 114, 148, serum 89, 93, 94, 95, 148, CSF 54, 149, 150, 151, brain 15, 87, 109, 118, 132, 149, 152, heart 132, liver 87, 132 kidney 132, and lenses 153 is HPLC–FD. Due to very low fluorescence generated by Kyna the accurate quantification of nanomolar concentrations in biological samples requires signal improvement, i.e. by specific chelation with zinc ions 14. The postcolumn 14, 114, 117, 118, 149, 150, 151, 154 or on‐column 26, 40, 44, 81, 89, 93, 94, 109, 153 derivatization is utilized to obtain the fluorescent complex of Kyna with Zn2+. For this purpose, the Zn‐containing mobile phases with pH around 6.2 are used and allow for generation of their most reliable derivatives 114. It was also observed that an addition of Zn2+ could improve the chromatographic separation of Kyna and Trp and cause a some enhancement of Trp fluorescence signal 14. Increase of Kyna fluorescence is achieved by addition of 50 mM ammonium acetate or ammonium formate. They improve ionization of carboxyl group and in consequence increase formation of Kyna‐Zn2+ coordination complex 114. In most HPLC–FD methods, authors applied the mobile phases containing from 0.1 to 0.5 M zinc acetate. This high concentration of salt that is weakly soluble in mobile phase may cause particle crystallization thus caution needs to be taken 89, 93. It is recommended to acidify the mobile phase with acetic acid to reduce a risk of column clotting 26, 81, 99, 109, 114, 117, 118, although low pH has negative impact on Kyna‐Zn2+ complex formation and reduces its fluorescence 14, 114. Moreover, several endogenous compounds present in a biological sample might also interfere with the complex formation 118. Finally, due to the shorter a retention time of Kyna on ODS column, the mobile phase containing acetonitrile is frequently used as shown in Table 3.
Table 3.
Chromatographic methods for Kyna determination
| HPLC‐UV | |||||
|---|---|---|---|---|---|
| LOD(nM) | CR (μM) | Mobile phase composition | λ (nm) | Application | Reference |
| − | up to 528.64 | 40 mM acetate‐citrate buffer (pH 4.5), 2.5% v/v CH3CN | 254 | Human urine | 123 |
| − | − | A: 50 mM CH3COONa (pH 4.8), B: 50 mM CH3COONa (pH 3.65), C: 100% v/v CH3CN, D: 100% v/v MeOH | 330 | Human serum | 41 |
| HPLC‐FD | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | λex/λem (nm) | Application | Reference |
| 0.9 | 0.001 – 0.10 | 20 mM CH3COONa, 3 mM (CH3COO)2Zn, 7% v/v CH3CN | 344/398 | Human plasma | 14 |
| 0.002 | − | 100 mM (CH3COO)2Zn, 50 mM CH3COOH, 3% v/v CH3CN | 344/390 | Human plasma | 44, 46 |
| − | − | 250 mM (CH3COO)2Zn, 0.9% v/v CH3CN (pH adjusted to 5.8 with CH3COOH) | 334/388 | Human plasma | 40 |
| 0.0005 | 0.00 – 1.00 | 50 mM CH3COOH, 250 mM (CH3COO)2Zn (pH 4.9), 1% v/v CH3CN | 254/404 | Human serum | 94 |
| 0.002 | − | 100 mM (CH3COO)2Zn, 50 mM CH3COOH, 3% v/v CH3CN | 344/390 | Human plasma | 26 |
| 0.011 | 2.62 – 1047.00 | 250 mM (CH3COO)2Zn, 3% v/v CH3CN | 344/404 | Human serum | 89 |
| 0.00005 | 0.002– 2.09 | 50 mM CH3COONa, 500 mM (CH3COO)2Zn, 6% v/v CH3CN | 344/404 | Human serum | 93 |
| 0.00008 | − | 0.1 CH3COO, 5% v/v CH3CN | 251/398 | Human serum | 117 |
| − | − | 50 mM CH3COOH, 50 mM (CH3COO)2Zn (pH 4.9), 1.2% v/v CH3CN | 254/404 | Rat plasma | 81 |
| 0.00016 | 0.025 – 0.20 | 0.1M CH3COOH, 5% v/v CH3CN | 251/398 | Rat plasma | 114 |
| 0.0017 | − | A: 60% v/v 0.5 M (CH3COO)2Zn 2, B: 40%: v/v 0.1 M CH3COONa, 40% v/v CH3CN, pH adjusted to 6.3 with CH3COOH | 344/389 | Rat plasma | 109 |
| − | − | 5 mM CH3COONa, 250 mM (CH3COO)2Zn, 3% v/v CH3CN, pH adjusted to 6.2 with CH3COOH | 344/398 |
|
87 |
| − | − |
250 mM (CH3COO)2Zn, 50 mM CH3COONa, 3% v/v CH3CN pH adjusted to 6.2 with CH3COOH |
344/398 |
|
61 |
| − | − | 250 mM (CH3COO)2Zn, 50 nM CH3COONa, 2.25% v/v CH3CN | 344/388 | Human CFS | 54 |
| 0.0001 | − | 90% v/v H2O, 10% v/v CH3CN, 0.1% TCA | 330/390 |
|
152 |
| HPLC‐ED | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | Application | Reference | |
| 0.027 | 0.003– 79.29 | 50 mM sodium phosphate‐acetate buffer (pH 4.1), 10% v/v MeOH, 0.42 mM octanesulphonic acid | Rat brain | 91 | |
| LC‐MS/MS | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | Monitored ions / transitions (m/z) | Application | Reference |
| 0.0034 | 0.006 – 95.00 | A: 2.1% v/v HCOOH (pH 2.0), B: 2.1% v/v HCOOH, 40% v/v CH3CN, C: 2.1% v/v HCOOH, 90% v/v CH3CN | 206>189 | Human plasma | 99 |
| 0.004 | 0.0005 – 0.40 | A: 650 mM CH3COOH, B: 100 mM heptafluorobutyric acid, C: 90% v/v CH3CN in H2O | 190.3>143.9 | Human plasma | 86 |
| 0.0180 | 0.07 – 1.12 | A: HCOONH4 in H2O (0.05% v/v), pH adjusted to 5.5 with CH3COOH, B: 100% v/v CH3CN | 189.9>143.7>89 | Rat plasma | 108 |
|
|
A: 0.1% v/v CH3COOH, B: 100% v/v MeOH | 190>144>172 |
|
2 |
| 0.0003 | 0.001 – 5.00 | A: 0.1% v/v HCOOH in H2O, B: 0.1% v/v HCOOH in CH3CN | 190.2>144 |
|
111 |
| 0.0095 | − | A: 25% v/v 0.4 M CH3COONH4, 32% v/v MeOH, 40% v/v CH3CN, B: 32% v/v MeOH, 68% v/v CH3CN | 383>387>389 | Cell culture medium | 127 |
| − | − | A: 0.1% v/v HCOOH in H2O, B: 0.1% v/v HCOOH in CH3CN | 190.2>144 | Rat hepatocytes | 110 |
| 0.012 | 0.01 – 1.71 | A: 20 nM CH3COONH4, 0.1% v/v HCOOH in H2O, B: 100% v/v CH3CN | 424>170 | Rat brain | 141 |
| GC‐MS/MS | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Monitored ions / transitions (m/z) | Application | Reference | |
| 0.0002 | − | 368>372>374 | Cell culture medium | 127 | |
LOD, limit of detection; CR, calibration range; λ, wavelength; λex, excitation wavelength; λem, emission wavelength.
It is also known that due to appearance of the carboxyl group in the chemical structure of Kyna its capacity factor (retention properties on a column) under the mobile phase at pH 6.2 is considerably small 149. To resolve this problem and to improve the separation of Kyna from unknown compounds present in biological samples, a postcolumn derivatization employing a column‐switching HPLC system has been proposed 114. The system consists of two ODS columns connected to trapping column (an anion‐exchange column). Kyna separation from interfering compounds is carried out with the first ODS column and an acidic mobile phase. The peak fraction of Kyna is next trapped on the anion‐exchange column and then is introduced into the second ODS column in the optimal mobile phase (pH 7.0) followed by fluorescence detection of Kyna‐Zn2+ complex. This column‐switch HPLC system was successfully applied for Kyna determination in 10 μL of a rat plasma. The sample before chromatographic separation was first deproteinated using 50 mM ammonium acetate in methanol. The detection limit of this method was about 0.16 nM. The method proposed by Mitsuhashi et al. after some improvements was applied to quantify Kyna in human serum 117. The detection limit of the improved column‐switching HPLC method was approximately 0.08 nM (4.0 fmol/injection, S/N = 3). Furthermore, the method required only 7.5 μL of human serum demonstrating its clinical usefulness. The same research group investigated the applicability of the column‐switch HPLC approach to rat brain homogenates after a simple pretreatment including deproteinization with acetone 118 and Zhao's group showed simultaneous determination of Kyn, Kyna, and Trp in human plasma 14.
Kyna level in biological samples might be measured also by LC coupled with MS detectors 2, 86, 111, 141. Amirkhani's group proposed a capillary liquid chromatography with ESI‐MS/MS method for simultaneous determination of Trp, Kyn and Kyna in human plasma 99. Finally, Kyna can be also measured as the pentafluorobenzyl derivative using GC–MS 127.
4.1.4. Determination of 3‐hydroxykynurenine
HPLC with electrochemical detection (ED) represents the widely used method for 3HKyn quantification 44, 61, 65, 81, 91, 155. The samples are first separated using different compositions of mobile phase including phosphoric acid, EDTA, triethylamine, heptane sulfonic acid, and addition of small amount of methanol or acetonitrile. Following separation on column, 3HKyn is detected by oxidation at potential in the range of 0.5 to 0.65 V. The obtained electrical current linearly correlates to the concentration of analyte. The electrochemical detection is known for its high sensitivity, however, the main drawback of this approach is lack of reproducibility caused by electrode clogging 141 and loss in selectivity. The last can be improved by optimization of separation conditions and removing the compounds that undergo reduction or oxidation at the same potential as target analyte. The HPLC–ED with chiral column presents also an advantage to separate 3HKyn enantiomers 87 and for this purpose variant of electrochemical detection of 3HKyn in conjunction with CMEK can be employed 143.
3HKyn shows specific absorbance at 365 nm and this property is utilized in its quantification by HPLC–UV 41, 54, 95, 112, 135, 156, while derivatization with p‐toluenesulfonyl chloride gives a fluorescent derivative analyzed by HPLC–FD 156. The other chromatographic methods of 3HKyn determination in different samples such as plasma 86, 108, 130, serum 2, 111, CSF 2, urine 111, 140, 141, cells 110, 111, 121, and culture medium 121 include several protocols of LC–MS/MS as well as GC–MS 119 after sample derivatization with pentafluoropropionic anhydride and 2,2,3,3,3‐pentafluoro‐1‐propanol, respectively. Comparison of different chromatographic protocols for 3HKyn determination is given in Table 4.
Table 4.
Chromatographic methods for 3HKyn determination
| HPLC‐UV | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | λ(nm) | Application | Reference |
|
0.00 – 100.00 | 50 mM CH3COOH, 250 mM (CH3COO)2Zn (pH 4.9), 1% v/v CH3CN |
|
Human serum | 94 |
| − | up to 446.00 | 40 mM acetate‐citrate buffer (pH 4.5), 2.5% v/v CH3CN | 254 | Human urine | 123 |
| − | − | 0.1 M CH3COONH4 (pH 4.65) | 365 | Human CFS | 54 |
| HPLC‐ED | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | Application | Reference | |
| 0.03 | − | 50 mM H3PO4, 50 mM citric acid, 60 μM EDTA, 8 mM heptane sulfonic acid, 2 mM NaCl, 5% v/v MeOH, pH adjusted to 3.1 with KOH | Human plasma | 46 | |
| − | − | 1.55 v/v CH3CN, 0.9% v/v triethylamine, 0.59% H3PO4, 0.27 M EDTA, 8.9 mM sodium heptanesulfonic acid |
|
61 | |
| − | − | 0.1 M triethylamine, 0.1 M H3PO4, 0.3 mM EDTA, 8.2 mM heptane‐1‐sulfonic acid sodium salt, 2% v/v CH3CN | Rat plasma | 81 | |
| − | − | 1.5% v/v CH3CN, 0.9% v/v triethylamine,0.59% v/v H3PO4, 0.27 mM EDTA, 8.9 mM sodium heptanesulfonic acid |
|
87 | |
| 0.0056 | 0.003 – 66.90 | 50 mM sodium phosphate‐acetate buffer (pH 4.1), 10% v/v MeOH, 0.42 mM octanesulphonic acid | Rat brain | 91 | |
| − | − | 0.1 M citrate buffer (pH 3.0), 8% v/v CH3CN, 0.4 mM octyl sulfate | Mosquito larval | 55 | |
| CMEKC‐ED | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | Application | Reference | |
| 0.0074 | 0.03 – 10.00 | 10 M Na2HPO4‐NaOH buffer (pH 11.0), 40 mM sodium dodecyl sulfate, 3% v/v MeOH | Rabbit urine | 143 | |
| LC‐MS/MS | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | Monitored ions / transitions (m/z) | Application | Reference |
| 0.002 | 0.0005 – 0.40 | A: 650 mM CH3COOH, B: 100 mM heptafluorobutyric acid, C: 90% v/v CH3CN in H2O | 225.2>208.3 | Human plasma | 86 |
| 0.005 | 0.02 – 45.00 | A: 0.2% v/v HCOOH, B: 100% v/v CH3CN | 225>110>208>162 | Human plasma | 130 |
| 0.04 | 0.08 – 1.35 | A: 0.05% v/v HCOONH4 inH2O, pH adjusted to 5.5 with CH3COOH, B: 100% v/v CH3CN | 153.82>135.8>79.8 | Rat plasma | 108 |
|
|
A: 0.1% v/v CH3COOH, B: 100% v/v MeOH | 225>110>162 |
|
2 |
| 0.009 | 0.009 – 2.23 | A: 0.1% v/v HCOOH in H2O, B: 0.1% v/v HCOOH in H2O | 691 | Human urine | 140 |
| 0.1 | 0.02 – 10.00 | A: 5 mM HCOONH4, 0.01% v/v TCA, B: 100% v/v MeOH | 225>110 |
|
121 |
| 0.001 | 0.002 – 10.00 | A: 0.1% v/v HCOOH in H2O, B: 0.1% v/v HCOOH in CH3CN | 225.2>208 |
|
111 |
| − | − | A: 0.1% v/v HCOOH in H2O, B: 0.1% v/v HCOOH in CH3CN | 225.2>208 | Rat hepatocytes | 110 |
| GC‐MS/MS | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Monitored ions/transitions (m/z) | Application | Reference | |
| − | 0.001 – 1.00 | 630.0>219.0 | Rat brain | 87 | |
LOD, limit of detection; CR, calibration range; λ, wavelength.
4.1.5. Determination of 3‐Hydroxyanthranilic Acid and Antrahilic Acid
The 3HAA accumulates in brain and different kinds of cells at nanomolar concentrations. To estimate the physiological role of 3HAA, it is assayed simultaneously with other kynurenines, mainly 3HKyn. The reported chromatographic methods for 3HAA determination are summarized in Table 5. The main approach used for this purpose is LC–MS 2, 86, 110, 111, 121, 127, 140 followed by HPLC coupled with fluorescence 40, 85, 94, 157, 158, UV 95, 135, or electrochemical 46, 85, 91 detectors. It is noticeable, that detection limit of 3HAA improved by 70% after addition of 0.01% TCA to the mobile phase 121.
Table 5.
Chromatographic approaches for 3HAA determination
| HPLC‐UV | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | λ (nm) | Application | Reference |
| − | up to 660.00 | 40 mM acetate‐citrate buffer (pH 4.5), 2.5% v/v CH3CN | 254 | Human urine | 123 |
| HPLC‐FD | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | λex/λem (nm) | Application | Reference |
| − | − | 250 mM (CH3COO)2Zn, 0.9% v/v CH3CN, pH adjusted to 5.8 with CH3COOH | 316/420 | Human plasma | 40 |
| 0.001 | 0.00–10.00 | 50 mM CH3COOH, 250 mM (CH3COO)2Zn (pH 4.9), 1% v/v CH3CN | 320/420 | Human serum | 94 |
| 0.0003 | up to 0.50 | 2 mM hexyl sodium sulfate, 1 mM EDTA, 8% v/v MeOH, 48 mM citric acid, 73 mM NaOH (pH 4.65) | 311/414 | Rat plasma | 157 |
| 0.0003 | up to 0.50 | 2 mM hexyl sodium sulfate, 1 mM EDTA, 1.5% v/v MeOH, 48 mM citric acid, 73 mM NaOH (pH 4.45) | 311/414 | Rat brain | 157 |
| 0.001 | 0.005–0.05 | 90% v/v sodium acetate buffer (pH 5.5), 10% v/v MeOH | 316/420 | Rat brain | 85 |
| HPLC‐ED | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | Application | Reference | |
| 0.030 | − |
|
Human plasma | 44, 46 | |
| 0.005 | 0.004–97.95 | 50 mM sodium phosphate‐acetate buffer (pH 4.1), 10% v/v MeOH, 0.42 mM octanesulphonic acid | Rat brain | 91 | |
| LC/MS or LC/MS‐MS | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR(μM) | Mobile phase composition | Monitored ions / transitions (m/z) | Application | Reference |
| 0.002 | 0.0005– 0.40 | A: 650 mM CH3COOH, B: 100 mM heptafluorobutyric acid. C: 90% v/v CH3CN in H2O | 154.1 > 80.0 | Human plasma | 86 |
|
|
A: 0.1% v/v CH3COOH, B: 100% MeOH | 154 > 136 > 108 |
|
2 |
| 0.1 | 0.10–10.00 | A: 5 mM CH3COONH4, 0.01% v/v TCA, B: 100% MeOH | 154 > 80 |
|
121 |
| 0.003 | 0.005–10.00 | A: 0.1% v/v HCOOH in H2O, B: 0.1% v/v HCOOH in CH3CN | 154.2 > 80.0 |
|
111 |
| 0.002 | 0.002–3.26 | A: 0.1% v/v HCOOH in H2O, B: 0.1% v/v HCOOH in H2O | 387 | Human urine | 140 |
| − | − | A: 0.1% v/v HCOOH in H2O, B: 0.1% v/v HCOOH in CH3CN | 154.2 > 80 | Rat hepatocytes | 110 |
| 0.003 | − | A: 28% v/v 0.4 M CH3COONH4, 32% v/v MeOH, 40% v/v CH3CN, B: 32% v/v MeOH, 68% v/v CH3CN | 383 > 387 > 389 | Culture medium | 127 |
LOD, limit of detection; CR, calibration range; λ, wavelength; λex, excitation wavelength; λem, emission wavelength.
Antrahilic acid is also present at nanomolar concentrations in plasma and brain 85, 108 and similar methodology utilizing HPLC–UV 95, 135, fluorescence 85, 110, LC–MS/MS 2, 108, 111, 143, as well as electrochemical 91 detectors are used for AA quantification as the method of choice for several analyses of urine, blood, and CSF. The lowest LOD was, however, achieved using GC with electron capture chemical ionization (ECNI) MS 127. The chromatographic methods proposed for AA quantification are presented in Table 6.
Table 6.
Chromatographic approaches for AA determination
| HPLC‐UV | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | λ (nm) | Application | Reference |
| − | up to 729.18 | 40 mM acetate‐citrate buffer (pH 4.5), 2.5% v/v CH3CN | 254 | Human urine | 123 |
| HPLC‐FD | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR(μM) | Mobile phase composition | λex/λem (nm) | Application | Reference |
| 0.001 | 0.005 – 0.05 | 90% v/v sodium acetate buffer (pH 5.5), 10% v/v MeOH | 316/420 | Rat brain | 85 |
| 0.015 | 0.01 – 0.44 | A: 0.1% v/v TCA in H2O, B: 0.1% v/v TCA in MeOH | 339/419 | Rat serum | 110 |
| HPLC‐ED | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR(μM) | Mobile phase composition | Application | Reference | |
| 0.014 | 0.004 – 109.38 | 50 mM sodium phosphate‐acetate buffer (pH 4.1), 10% v/v MeOH, 0.42 mM octanesulphonic acid | Rat brain | 87 | |
| LC/MS‐MS | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Mobile phase composition | Monitored ions/transitions (m/z) | Application | Reference |
| 0.007 | 0.0005 – 0.40 | A: 650 mM CH3COOH, B: 100 mM heptafluorobutyric acid, C: 90% v/v CH3CN in H2O | 138.1>120.1 | Human plasma | 86 |
| 0.024 | 0.09 – 1.50 | A: (0.05% v/v) CH3COONH4 in H2O, pH adjusted to 5.5 with CH3COOH, B: 100 % v/v CH3CN | 137.86 >119.9>91.9 | Rat plasma | 108 |
|
|
A: 0.1% v/v CH3COOH, B: 100% MeOH | 138>120>92 |
|
2 |
| 0.0004 | 0.001 – 1.00 | A: 0.1% v/v HCOOH in H2O, B: 0.1% v/v HCOOH in CH3CN | 138.2>120.0 |
|
111 |
| GC‐MS/MS | |||||
|---|---|---|---|---|---|
| LOD (μM) | CR (μM) | Monitored ions/transitions (m/z) | Application | Reference | |
| 0.0001 | − | 136>140>142 | Cell culture medium | 127 | |
LOD, limit of detection; CR, calibration range; λ, wavelength; λex, excitation wavelength; λem, emission wavelength.
4.1.6. Determination of quinolinic acid
Majority of methods describing quantification if Quin are based on detection with MS methodology. Several protocols utilizing GC for different biological samples, such as rat brain tissues 84, brain microglia cells, astrocytes, neurons from human fetus 159, whole blood and plasma 84 and different kind of cells 122, 159 have been described. Heyes et al. have proposed estimation of Quin by measuring the volatile derivative (dihexafluoroisopropyl ester) by GC–ECNI‐MS 84. In contrast, Nartisin's GC–ECNI‐MS protocol for simultaneous derivatization of trace concentrations of Quin and ʟ‐Trp, ʟ‐Kyn, Kyna, AA, XA, PIC can be used even without preseparation step 127. The method employs lyophilization of aqueous samples in the presence of tetrabutylammonium hydrogen sulfate followed by base‐catalyzed anhydrous pentafluorobenzylation. The pentafluorobenzyl derivatives of ʟ‐Trp and ʟ‐Kyn can be analyzed using GC–ECNI‐MS at impressively small quantities of femtogram amounts 127. In addition, authors have shown that GC–ECNI‐MS is more sensitive than LC/ECNI‐MS for XA and Quin determination. On the other hand, Eckstein and coworker have proposed a GC–MS/MS method with electron capture chemical ionization (GC–ECCI‐MS) for simultaneous monitoring of 43 amino acids and biogenic amines including Trp, Kyn, Kyna, 3HKyn, and XA in CSF 160. The method is based on sample derivatization with 2,2,3,3,3‐pentafluoro‐1‐propanol and pentafluoropropionic anhydride according to protocol described by Watson 161, and was applied after some modification by Notarangeloʼs group (e.g. they used ECNI detection) for determination of Quin and ʟ‐Trp, ʟ‐Kyn, 3HKyn in a sample of rat brain 119 or just Quin in brain, liver tissue, and plasma from mice 61. Moreover, sample derivatization with 2,2,3,3,3‐penta‐fluoro‐1‐propanol and pentafluoropropionic anhydride was used by Sano and coworkers for determination of Quin and other Trp metabolites in human and rat plasma using GC–ECNI‐MS 102. The femtomolar sensitivity was achieved by Smythe and coworkers measuring hexafluoroisopropyl esters on GC–ECNI‐MS for Quin, PIC, and nicotinic acid determination 162. Dobbie et al. have proposed GC electron impact (EI) MS analysis for Quin derivatized to di‐tert‐butyldimethylsilyl ester (tBDMS) 163. Ionization by EI‐MS generally results in better fragmentation compared to ECNI‐MS 126. On the other hand, the ECNI‐MS approach does not require prepurification of Quin nor cleaning up of the generated derivative 126 and has been used to study age‐related changes of Quin level in CSF from children and patients with Huntington's disease 44, as well as in blood samples from patients with chronic brain injury 46.
Alternatively, the LC–MS/MS methods have been also proposed for quantification of Quin in variety of biological samples, i.e. plasma 86, 108, serum 111, 129, urine 111, and cultured cells 111, 127. Sample derivatization improves Quin ionization response in the positive ESI mode and might be advantageous for small amount of sample 129. Unfortunately, the SPE is required to eliminate the interfering compounds 108, 129.
4.1.7. Determination of picolinic acid and xanthurenic acid
The PIC detection in serum 41, 83, plasma 108, 162, brain tissues 162, and culture medium 127, 162 have been reported using HPLC–UV, ion‐exchange chromatography 83, LC–MS/MS 108, and GC–ECNI‐MS 127, 162. The small‐volume sample analysis without compromising of sensitivity is achieved by derivatization using pentafluorobenzyl 127 or hexafluoroisopropyl 162 and GC–ECNI‐MS analysis. The fact that PIC can form complexes with proteins and bind to the resin makes HPLC method difficult to use for PIC determination 83, 129. The addition of ion‐pair reagent tetrabutylammonium hydrogen sulfate to a mobile phase has been found to improve the chromatographic elution of PIC 83.
The XA is frequently measured by LC coupled with MS 86, 110, 111, 127, UV 81, 123, or electrochemical 41, 46, 91 detectors. The buffers with pH about 4.6 are used for mobile phase and wavelength ranging from 254 to 338 nm has been applied for detection. The XA measurement can be also performed by GC–MS/MS 127.
4.2. Guides for choosing the most suitable chromatographic method for determination of kynurenines
While selecting the method for Trp metabolites assessment at first the expected concentration in the tested specimen should be considered, i.e. Kyn and HAA concentration in human serum is obviously lower (about 1.35 μM and 79 nM, respectively) than that of Trp (about 38 μM) 94. Further, while Trp and Kyn are present in human hippocampus at concentrations about 110 and 20 nM/g tissue, respectively 15, 164, HAA concentration level in rat hippocampus is about 12 fM/g 85. It is also very important to establish, whether the simultaneous analysis of multiple compound is aimed. The most sensitive and selective for this purpose seems to be LC–MS and LC–MS/MS and they are being increasingly used by many researchers for determination of multiple kynurenines. However, these protocols require an expensive equipment, careful sample preparation (including SPE or derivatization) as well as costly isotope‐labeled internal standards. HPLC systems equipped in various types of detectors (UV, fluorescence) are more accessible for analysis in clinical and simple laboratory settings. The HPLC with the UV detector is mainly used for Kyn quantification, although HPLC–UV methods suffer from low sensitivity and selectivity due to an interference of endogenous compounds present in biological samples. The improvement is observed when using HPLC–FD, but the main drawback is that not all kynurenines show fluorescence (e.g. Kyn) or their native fluorescence is too low for accurate quantification without derivatization in complex samples. This methodology is predominantly chosen for Kyna detection. In case of ED detection, the selectivity and repeatability is compromised due to interference of sample components and the electrode clogging, respectively. The HPLC–ED is often used for 3HKyna and 3HAA detection, while the GC–MS or GC–MS/MS is better suited for Quin and PIC due to their low concentration in tissue. These GC assays require expensive equipment and precise sample preparation including purification step and derivatization of target analytes into volatile derivatives. The advantage presented by these methods is high sensitivity and small amount of sample required for analysis. The quick guide for choosing of the optimal approach is summarized in Fig. 2.
Figure 2.
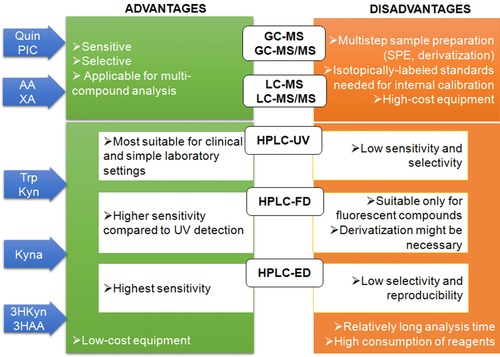
Comparison of chromatographic methods applied for analysis of Trp metabolites
5. CONCLUDING REMARKS
The involvement of endogenous toxins in the disease mechanism is intensively studied in context of neurodegenerative disorders, cancer, viral infections, or immune regulation. The kynurenine pathway metabolites that include neurotoxic as well as neuroprotective compounds have been widely investigated in this context. Although, a lot of work has been done there is still need to better understand the balance between the level of different Trp metabolites and consequences in organism. These goals cannot be achieved without efficient analytical protocols for accurate and fast determination of kynurenines in body fluids, tissues, and cultured cells. As a result, large number of studies dealing with protocols for kynurenines determination can be found in the literature. Majority of the applied methods regard the chromatographic approach combined with diverse detection modes, including UV, fluorescence, or different types of MS. This diversity of possible methods might be discouraging for many researchers trying to choose the optimal protocol. The issues regarding sample amount, complex composition, fast analytes decomposition, differences in quantity distribution of various kynurenines in the studied sample and amounts present in different tissues should be considered when choosing the chromatographic method.
In future studies aimed to understand the role of Trp metabolites in pathological states, a fast and direct quantification of all kynurenines during a single analytical run would be an ideal solution. This could be achieved with the universal detector allowing for establishing a consistent relationship between the magnitude of response and quantity of target analytes present in the sample. In case of Trp‐derived products LC method is favored, however, as was demonstrated above, no single LC‐coupled detector (UV/visible, fluorescence, electrochemical, mass spectrometric) is capable to detect all kynurenines at physiological level in a given chromatographic eluent. Therefore, future developments in the field of detection technology regarding modern LC are desired. The mass spectrometric detectors are the closest to being called universal detectors of kynurenines. The chromatographic systems coupled with these "near‐universal" detectors can serve as high performance quantification of Trp metabolites by both LC and GC approaches. Moreover, these methodologies require a small injection volume, what is important in case of limited sample amount. On the other hand, these systems are still not included in the basic equipment of laboratories because of their cost and requirement of highly skilled personnel. Therefore, much simpler HPLC systems equipped with UV/visible detectors are mainly utilized. These methods for the multikynurenines analysis unfortunately compromise selectivity and sensitivity. To overcome this problem further improvements in column design and separation conditions should be worked out.
Finally, there is also a need for methods directed at quantification of different enantiomers of Trp and kynurenines. The knowledge on relationship between the ᴅ and ʟ forms of Trp metabolites is important for understanding their involvement in disease mechanism. Currently, this is limited to the first products of the kynurenine pathway (ᴅ‐Trp, ᴅ‐Kyn), thus new methodologies for separation and measurement of chiral compounds must be developed.
ACKNOWLEDGMENTS
Authors thank Dr Zeina Dagher from Massachusetts General Hospital, Harvard Medical School, Boston, MA for help with review of the manuscript. This work was supported by the Operational Programme Development of Eastern Poland 2007–2013 (agreement POPW.01.03.00‐06‐003/09‐00) and the grant from The Leading National Research Centre (KNOW) for years 2014‐2018 (42/2016/KNOW/IITD).
Sadok I, Gamian A, Staniszewska Maria M. Chromatographic analysis of tryptophan metabolites. J Sep Sci. 2017;40:3020–3045. https://doi.org/10.1002/jssc.201700184
Conflict of interests: Authors declare no conflict of interest.
REFERENCES
- 1. Badawy, A. , The tryptophan utilization concept in pregnancy. Obstet. Gynecol. Sci. 2014, 57, 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Henykova, E. , Vranova, H. P. , Amakorova, P. , Pospisil, T. , Zukauskaite, A. , Vlckova, M. , Stable isotope dilution ultra‐high performance liquid chromatography‐tandem mass spectrometry quantitative profiling of tryptophan‐related neuroactive substances in human serum and cerebrospinal fluid. J. Chromatogr. A 2016, 1437, 145–157. [DOI] [PubMed] [Google Scholar]
- 3. Pertovaara, M. , Raitala, A. , Uusitalo, H. , Pukander, J. , Helin, H. , Oja, S. S. , Hurme, M. , Mechanisms dependent on tryptophan catabolism regulate immune responses in primary Sjögren's syndrome. Clin. Exp. Immunol. 2005, 142, 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grant, R. S. , Coggan, S. E. , Smythe, G.A. , The physiological action of picolinic acid in the human brain. Int. J. Tryptophan Res. 2009, 2, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ren, S. , Correia, M. , Heme: a regulator of rat hepatic tryptophan 2,3‐dioxygenase? Arch. Biochem. Biophys. 2000, 377, 195–203. [DOI] [PubMed] [Google Scholar]
- 6. Watanabe, Y. , Yoshida, R. , Sono, M. , Hayaishi, O. , Immunohistochemical localization of indoleamine 2,3‐dioxygenase in the argyrophilic cells of rabbit duodenum and thyroid gland. J. Histochem. Cytochem. 1981, 29, 623–632. [DOI] [PubMed] [Google Scholar]
- 7. Babcock, T. , Carlin, J. M. , Transcriptional activation of indoleamine dioxygenase by interleukin 1 and tumor necrosis factor alpha in interferon‐treated epithelial cells. Cytokine 2000, 12, 588–594. [DOI] [PubMed] [Google Scholar]
- 8. Widner, B. , Sepp, N. , Kowald, E. , Ortner, U. , Wirleitner, B. , Fritsch, P. , Baier‐Bitterlich, G. , Fuchs, D. , Enhanced tryptophan degradation in systemic lupus erythematosus. Immunobiology 2000, 201, 621–630. [DOI] [PubMed] [Google Scholar]
- 9. Kwidzinski, E. , Bunse, J. , Aktas, O. , Richter, D. , Mutlu, L. , Zipp, F. , Nitsch, R. , Bechmann, I. , Indolamine 2,3‐dioxygenase is expressed in the CNS and down‐regulates autoimmune inflammation, FASEB J. 2005, 19, 1347–1349. [DOI] [PubMed] [Google Scholar]
- 10. Tabebordbar, M. , Zhu, K. , Cheng, J. K. W. , Chew, W. L. , Widrick, J. J. , Yan, W. X. , Maesner, C. , Wu, E. Y. , Xiao, R. , Ran, F. A. , Cong, L. , Zhang, F. , Vandenberghe, L. H. , Church, G. M. , Wagers, A. J. , In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 2016, 351, 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mellor, A. L. , Munn, D. H. , Tryptophan catabolism and regulation of adaptive immunity. J. Immunol. 2003, 170, 5809–5813. [DOI] [PubMed] [Google Scholar]
- 12. Frumento, G. , Rotondo, R. , Tonetti, M. , Damonte, G. , Benatti, U. , Ferrara, G. B. , Tryptophan‐derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3‐dioxygenase. J. Exp. Med. 2002, 196, 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hwu, P. , Du, M. X. , Lapointe, R. , Do, M. , Taylor, M. W. , Young, H. , Indoleamine 2,3‐dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J. Immunol. 2000, 164, 3596–3599. [DOI] [PubMed] [Google Scholar]
- 14. Zhao, J. , Gao, P. , Zhu, D. , Optimization of Zn2+‐containing mobile phase for simultaneous determination of kynurenine, kynurenic acid and tryptophan in human plasma by high performance liquid chromatography. J. Chromatogr. B 2010, 878, 603–608. [DOI] [PubMed] [Google Scholar]
- 15. Baran, H. , Jellinger, K. , Deecke, L. , Kynurenine metabolism in Alzheimer's disease. J. Neural Transm. 1999, 106, 165–181. [DOI] [PubMed] [Google Scholar]
- 16. Rossi, F. , Han, Q. , Li, J. , Li, J. , Rizzi, M. , Crystal structure of human kynurenine aminotransferase I. J. Biol. Chem. 2004, 279, 50214–50220. [DOI] [PubMed] [Google Scholar]
- 17. Han, Q. , Robinson, H. , Li, J. , Crystal structure of human kynurenine aminotransferase II. J. Biol. Chem. 2008, 283, 3567–3573. [DOI] [PubMed] [Google Scholar]
- 18. Guidetti, P. , Hoffman, G. E. , Melendez‐Ferro, M. , Albuquerque, E.X. , Schwarcz, R. , Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia 2007, 55, 78–92. [DOI] [PubMed] [Google Scholar]
- 19. Parrott, J. M. , O'Connor, J. C. , Kynurenine 3‐monooxygenase: an influential mediator of neuropathology. Front. Psychiatry 2015, 6, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ligam, P. , Manuelpillai, U. , Wallace, E. M. , Walker, D. , Localisation of indoleamine 2,3‐dioxygenase and kynurenine hydroxylase in the human placenta and decidua: Implications for role of the kynurenine pathway in pregnancy. Placenta 2005, 26, 498–504. [DOI] [PubMed] [Google Scholar]
- 21. Moroni, F. , Tryptophan metabolism and brain function: focus on kynurenine and other indole metabolites. Eur. J. Pharmacol. 1999, 375, 87–100. [DOI] [PubMed] [Google Scholar]
- 22. Giulian, D. , Vaca, K. , Corpuz, M. , Brain glia release factors with opposing actions upon neuronal survival. J. Neurosci. 1993, 13, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koola, M. M. , Kynurenine pathway and cognitive impairments in schizophrenia: pharmacogenetics of galantamine and memantine. Schizophr. Res. Cogn. 2016, 4, 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davies, N. W. S. , Guillemin, G. , Brew, B. J. , Tryptophan, neurodegeneration and HIV‐associated neurocognitive disorder. Int. J. Tryptophan Res. 2010, 3, 121–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Opitz, C. A. , Wick, W. , Steinman, L. , Platten, M. , Tryptophan degradation in autoimmune diseases. Cell. Mol. Life Sci. 2007, 64, 2542–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Forrest, C. M. , Mackay, G. M. , Stoy, N. , Stone, T. W. , Darlington, L. G. , Inflammatory status and kynurenine metabolism in rheumatoid arthritis treated with melatonin. Br. J. Clin. Pharmacol. 2007, 64, 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williams, R. O. , Exploitation of the IDO pathway in the therapy of rheumatoid arthritis. Int. J. Tryptophan Res. 2013, 6, 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mancuso, R. , Hernis, A. , Agostini, S. , Rovaris, M. , Caputo, D. , Fuchs, D. , Clerici, M. , Indoleamine 2,3 dioxygenase (IDO) expression and activity in relapsing‐remitting multiple sclerosis. PLoS One 2015, 10, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cozzi, A. , Zignego, A. , Carpendo, R. , Biagiotti, T. , Aldinucci, A. , Monti, M. , Giannini, C. , Rosselli, M. , Laffi, G. , Moroni, F. , Low serum tryptophan levels, reduced macrophage IDO activity and high frequency of psychopathology in HCV patients. J. Viral Hepat. 2006, 13, 402–408. [DOI] [PubMed] [Google Scholar]
- 30. Larrea, E. , Riezu‐Boj, J. I. , Gil‐Guerrero, L. , Casares, N. , Aldabe, R. , Sarobe, P. , Civeira, M. P. , Heeney, J. L. , Rollier, C. , Verstrepen, B. , Wakita, T. , Borras‐Cuesta, F. , Lasarte, J. J. , Prieto, J. , Upregulation of indoleamine 2,3‐dioxygenase in hepatitis C virus infection. J. Virol. 2007, 81, 3662–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fernandez‐Pol, J. A. , Klos, D. J. , Hamilton, P. D. , Antiviral, cytotoxic and apoptotic activities of picolinic acid on human immunodeficiency virus‐1 and human herpes simplex virus‐2 infected cells. Anticancer Res. 2001, 21, 3773–3776. [PubMed] [Google Scholar]
- 32. Schröcksnadel, H. , Baier‐Bitterlich, G. , Dapunt, O. , Wachter, H. , Fuchs, D. , Decreased plasma tryptophan in pregnancy. Obstet. Gynecol. 1996, 88, 47–50. [DOI] [PubMed] [Google Scholar]
- 33. Munn, B. D. H. , Shafizadeh, E. , Attwood, J. T. , Bondarev, I. , Pashine, A. , Mellor, A. L. , Inhibition of T cell proliferation by macrophage. J. Exp. Med. 1999, 189, 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Munn, D. H. , Zhou, M. , Attwood, J. T. , Bondarev, I. , Conway, S. J. , Marshall, B. , Brown, C. , Mellor, A. L. , Medawar, P. B. , Tafuri, A. , Alferink, J. , Möller, P. , Hämmerling, G. J. , Arnold, B. , Munn, D. H. , Armstrong, E. , Munn, D. H. , Pressey, J. , Beall, A. C. , Hudes, R. , Alderson, M. R. , Kamimura, S. , Eguchi, K. , Yonezawa, M. , Sekiba, K. , Schrocksnadel, H. , Baier‐Bitterlich, G. , Dapunt, O. , Wachter, H. , Fuchs, D. , Cady, S. G. , Sono, M. , Spanopoulou, E. , Tarazona, R. , Bonney, E. A. , Matzinger, P. , Pollard, W. J. , Habara‐Ohkubo, A. , Takikawa, O. , Yoshida, R. , Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998, 281, 1191–1193. [DOI] [PubMed] [Google Scholar]
- 35. Kudo, Y. , Boyd, C. A. R. , Sargent, I. L. , Redman, C. W. G. , Tryptophan degradation by human placental indoleamine 2,3‐dioxygenase regulates lymphocyte proliferation. J. Physiol. 2001, 535, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. von Rango, U. , Krusche, C. A. , Beier, H. M. , Classen‐Linke, I. , Indoleamine‐dioxygenase is expressed in human decidua at the time maternal tolerance is established. J. Reprod. Immunol. 2007, 74, 34–45. [DOI] [PubMed] [Google Scholar]
- 37. Badawy, A. , Namboodiri, A. M. , Moffett, J. R. , The end of the road for the tryptophan depletion concept in pregnancy and infection. Clin. Sci. 2016, 130, 1327–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kudo, Y. , Boyd, C. A. R. , Sargent, I. L. , Redman, C. W. G. , Decreased tryptophan catabolism by placental indoleamine 2,3‐dioxygenase in preeclampsia. Am. J. Obstet. Gynecol. 2003, 188, 719–726. [DOI] [PubMed] [Google Scholar]
- 39. Kohl, C. , Walch, T. , Huber, R. , Kemmler, G. , Neurauter, G. , Fuchs, D. , Sölder, E. , Schröcksnadel, H. , Sperner‐Unterweger, B. , Measurement of tryptophan, kynurenine and neopterin in women with and without postpartum blues. J. Affect. Disord. 2005, 86, 135–142. [DOI] [PubMed] [Google Scholar]
- 40. Myint, A.‐M. , Kim, Y. K. , Verkerk, R. , Scharpé, S. , Steinbusch, H. , Leonard, B. , Kynurenine pathway in major depression: evidence of impaired neuroprotection. J. Affect. Disord. 2007, 98, 143–151. [DOI] [PubMed] [Google Scholar]
- 41. Schwarz, M. J. , Guillemin, G. J. , Teipel, S. J. , Buerger, K. , Hampel, H. , Increased 3‐hydroxykynurenine serum concentrations differentiate Alzheimer's disease patients from controls. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263, 345–352. [DOI] [PubMed] [Google Scholar]
- 42. Guillemin, G. J. , Brew, B. J. , Noonan, C. E. , Takikawa, O. , Cullen, K. M. , Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer's disease hippocampus. Neuropathol. Appl. Neurobiol. 2005, 31, 395–404. [DOI] [PubMed] [Google Scholar]
- 43. Ting, K. K. , Brew, B. J. , Guillemin, G. J. , Effect of quinolinic acid on human astrocytes morphology and functions: implications in Alzheimer's disease. J. Neuroinflammation 2009, 6, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stoy, N. , Mackay, G. M. , Forrest, C. M. , Christofides, J. , Egerton, M. , Stone, T. W. , Darlington, L. G. , Tryptophan metabolism and oxidative stress in patients with Huntington's disease. J. Neurochem. 2005, 93, 611–623. [DOI] [PubMed] [Google Scholar]
- 45. Widner, B. , Leblhuber, F. , Fuchs, D. , Increased neopterin production and tryptophan degradation in advanced Parkinson's disease. J. Neural. Transm. 2002, 109, 181–189. [DOI] [PubMed] [Google Scholar]
- 46. Mackay, G. M. , Forrest, C. M. , Stoy, N. , Christofides, J. , Egerton, M. , Stone, T. W. , Darlington, L. G. , Tryptophan metabolism and oxidative stress in patients with chronic brain injury. Eur. J. Neurol. 2006, 13, 30–42. [DOI] [PubMed] [Google Scholar]
- 47. Sinz, E. H. , Kochanek, P. M. , Heyes, M. P. , Wisniewski, S. R. , Bell, M. J. , Clark, R. S. , DeKosky, S. T. , Blight, A. R. , Marion, D. W. , Quinolinic acid is increased in CSF and associated with mortality after traumatic brain injury in humans. J. Cereb. Blood Flow Metab. 1998, 18, 610–615. [DOI] [PubMed] [Google Scholar]
- 48. Olajossy, M. , Potembska, E. , Skoczeń, N. , Olajossy, B. , Urbańska, E. , Changes in the levels of kynurenic acid and selected proinflammatory cytokines after pharmacological treatment and electroconvulsive therapy (ECT) in patients with depressive disorder. Curr. Probl. Psychiatry 2016, 17, 75–82. [Google Scholar]
- 49. Heyes, M. P. , Saito, K. , Milstien, S. , Schiff, S. J. , Quinolinic acid in tumors, hemorrhage and bacterial infections of the central nervous system in children. J. Neurol. Sci. 1995, 133, 112–118. [DOI] [PubMed] [Google Scholar]
- 50. Curto, M. , Lionetto, L. , Negro, A. , Capi, M. , Fazio, F. , Giamberardino, M. A. , Simmaco, M. , Nicoletti, F. , Martelletti, P. , Altered kynurenine pathway metabolites in serum of chronic migraine patients. J. Headache Pain 2015, 17, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen, Y. , Stankovic, R. , Cullen, K. M. , Meininger, V. , Garner, B. , Coggan, S. , Grant, R. , Brew, B. J. , Guillemin, G. J. , The kynurenine pathway and inflammation in amyotrophic lateral sclerosis. Neurotox Res. 2010, 18, 132–142. [DOI] [PubMed] [Google Scholar]
- 52. Miller, C. L. , Llenos, I. C. , Dulay, J. R. , Barillo, M. M. , Yolken, R. H. , Weis, S. , Expression of the kynurenine pathway enzyme tryptophan 2,3‐dioxygenase is increased in the frontal cortex of individuals with schizophrenia. Neurobiol. Dis. 2004, 15, 618–629. [DOI] [PubMed] [Google Scholar]
- 53. Schröcksnadel, K. , Wirleitner, B. , Winkler, C. , Fuchs, D. , Monitoring tryptophan metabolism in chronic immune activation. Clin. Chim. Acta 2006, 364, 82–90. [DOI] [PubMed] [Google Scholar]
- 54. de Bie, J. , Guest, J. , Guillemin, G. , Grant, R. , Central kynurenine pathway shift with age in women. J. Neurochem. 2016, 136, 995–1003. [DOI] [PubMed] [Google Scholar]
- 55. Guillemin, G. J. , Wang, L. , Brew, B. J. , Quinolinic acid selectively induces apoptosis of human astrocytes: potential role in AIDS dementia complex. J. Neuroinflammation 2005, 2, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Foster, A. C. , Vezzani, A. , French, E. D. , Schwarcz, R. , Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci. Lett. 1984, 48, 273–278. [DOI] [PubMed] [Google Scholar]
- 57. Beal, M. F. , Matson, W. R. , Swartz, K. J. , Gamache, P. H. , Bird, E. D. , Kynurenine pathway measurements in Huntington's disease striatum: evidence for reduced formation of kynurenic acid. J. Neurochem. 1990, 55, 1327–1339. [DOI] [PubMed] [Google Scholar]
- 58. Lee, D. Y. , Lee, K. S. , Lee, H. J. , Noh, Y. H. , Kim, D. H. , Lee, J. Y. , Cho, S. H. , Yoon, O. J. , Lee, W. B. , Kim, K. Y. , Chung, Y. H. , Kim, S. S. , Kynurenic acid attenuates MPP+‐induced dopaminergic neuronal cell death via a Bax‐mediated mitochondrial pathway. Eur. J. Cell Biol. 2008, 87, 389–397. [DOI] [PubMed] [Google Scholar]
- 59. Song, Z. , Ogaya, T. , Ishii, K. , Ichiba, H. , Izuka, H. , Fukushima, T. , Utilization of kynurenic acid produced from D‐kynurenine in an in vitro assay of D‐amino acid oxidase activity. J. Heal. Sci. 2010, 56, 341–346. [Google Scholar]
- 60. Fukushima, T. , Sone, Y. , Mitsuhashi, S. , Tomiya, M. , Toyo'Oka, T. , Alteration of kynurenic acid concentration in rat plasma following optically pure kynurenine administration: a comparative study between enantiomers. Chirality 2009, 21, 468–472. [DOI] [PubMed] [Google Scholar]
- 61. Notarangelo, F. , Wang, X.‐D. , Horning, K. , Schwarch, R. , Role of D‐amino acid oxidase in the production of kynurenine pathway metabolites from D‐tryptophan in mice. J. Neurosci. 2016, 136, 804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Okuda, S. , Nishiyama, N. , Saito, H. , Katsuki, H. , Hydrogen peroxide‐mediated neuronal cell death induced by an endogenous neurotoxin, 3‐hydroxykynurenine. Proc. Natl. Acad. Sci. USA 1996, 93, 12553–12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Eastman, C. L. , Guilarte, T. R. , Cytotoxicity of 3‐hydroxykynurenine in a neuronal hybrid cell line. Brain Res. 1989, 495, 225–231. [DOI] [PubMed] [Google Scholar]
- 64. Bonda, D , Mailankot, M , Stone, J , Garrett, M. S. M. , Castellani, R. J. , Siedlak, S. L. , Zhu, X. , Lee, H. G. , Perry, G. , Nagaraj, R. H. , Smith, M. A. , Indoleamine 2,3‐dioxygenase and 3‐hydroxykynurenine modifications are found in the neuropathology of Alzheimer's disease. Redox Rep. 2010, 15, 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pearson, S. J. , Reynolds, G. P. , Determination of 3‐hydroxykynurenine in human brain and plasma by high‐performance liquid chromatography with electrochemical detection. Increased concentrations in hepatic encephalopathy. J. Chromatogr. B Biomed. Sci. Appl. 1991, 565, 436–440. [DOI] [PubMed] [Google Scholar]
- 66. Muller, A. J. , DuHadaway, J. B. , Donover, P. S. , Sutanto‐Ward, E. , Prendergast, G. C. , Inhibition of indoleamine 2,3‐dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 2005, 11, 312–319. [DOI] [PubMed] [Google Scholar]
- 67. Opitz, C. A. , Litzenburger, U. M. , Sahm, F. , Ott, M. , Tritschler, I. , Trump, S. , Schumacher, T. , Jestaedt, L. , Schrenk, D. , Weller, M. , Jugold, M. , Guillemin, G. J. , Miller, C. L. , Lutz, C. , Radlwimmer, B. , Lehmann, I. , von Deimling, A. , Wick, W. , Platten, M. , An endogenous tumour‐promoting ligand of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203. [DOI] [PubMed] [Google Scholar]
- 68. Moon, Y. W. , Hajjar, J. , Hwu, P. , Naing, A. , Targeting the indoleamine 2,3‐dioxygenase pathway in cancer. J. Immunother. cancer 2015, 3, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen, J.‐Y. , Li, C.‐F. , Kuo, C.‐C. , Tsai, K. K. , Hou, M.‐F. , Hung, W.‐C. , Cancer/stroma interplay via cyclooxygenase‐2 and indoleamine 2,3‐dioxygenase promotes breast cancer progression. Breast Cancer Res. 2014, 16, 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Heng, B. , Lim, C. K. , Lovejoy, D. B. , Bessede, A. , Gluch, L. , Guillemin, G. J. , Understanding the role of the kynurenine pathway in human breast cancer immunobiology. Oncotarget 2015, 7, 6506–6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Platten, M. , Wick, W. , Van Den Eynde, B. J. , Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. 2012, 72, 5435–5440. [DOI] [PubMed] [Google Scholar]
- 72. Pilotte, L. , Larrieu, P. , Stroobant, V. , Colau, D. , Dolusic, E. , Frederick, R. , De Plaen, E. , Uyttenhove, C. , Wouters, J. , Masereel, B. , van den Eynde, B. J. , Reversal of tumoral immune resistance by inhibition of tryptophan 2,3‐dioxygenase. Proc. Natl. Acad. Sci. 2012, 109, 2497–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. NewLink Genetics Corporation , IDO inhibitor study for relapsed or refractory solid tumors (D‐1MT). ClinicalTrials.gov 2008.
- 74. Lob, S. , Konigsrainer, A. , Schafer, R. , Rammensee, H.G. , Opelz, G. , Terness, P. , Levo‐ but not dextro‐1‐methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood 2008, 111, 2152–2154. [DOI] [PubMed] [Google Scholar]
- 75. Finley, E. L. , Dillon, J. , Crouch, R. K. , Schey, K. L. , Identification of tryptophan oxidation products in bovine alpha‐crystallin. Protein Sci. 1998, 7, 2391–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mailankot, M. , Staniszewska, M. M. , Butler, H. , Caprara, M. H. , Howell, S. , Wang, B. , Doller, C. , Reneker, L. W. , Nagaraj, R. H. , Indoleamine 2,3‐dioxygenase overexpression causes kynurenine‐modification of proteins, fiber cell apoptosis and cataract formation in the mouse lens. Lab. Invest. 2009, 89, 498–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Staniszewska, M. M. , Nagaraj, R. H. , 3‐hydroxykynurenine‐mediated modification of human lens proteins: structure determination of a major modification using a monoclonal antibody. J. Biol. Chem. 2005, 280, 22154–22164. [DOI] [PubMed] [Google Scholar]
- 78. Staniszewska, M. , Nagaraj, R. H. , Detection of kynurenine modifications in proteins using a monoclonal antibody. J. Immunol. Methods 2007, 324, 63–73. [DOI] [PubMed] [Google Scholar]
- 79. Sheipouri, D. , Grant, R. , Bustamante, S. , Lovejoy, D. , Guillemin, G. J. , Braidy, N. , Characterisation of the kynurenine pathway in skin‐derived fibroblasts and keratinocytes. J. Cell. Biochem. 2015, 116, 903–922. [DOI] [PubMed] [Google Scholar]
- 80. Tsentalovich, Y. P. , Sherin, P. S. , Kopylova, L. V. , Cherepanov, I. V. , Grilj, J. , Vauthey, E. , Photochemical properties of UV filter molecules of the human eye. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7687–7696. [DOI] [PubMed] [Google Scholar]
- 81. Pawlak, D. , Tankiewicz, A. , Buczko, W. , Kynurenine and its metabolites in the rat with experimental renal insufficiency. J. Physiol. Pharmacol. 2001, 52, 755–766. [PubMed] [Google Scholar]
- 82. Bryleva, E. Y. , Brundin, L. , Kynurenine pathway metabolites and suicidality. Neuropharmacology 2017, 112, 324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dazzia, C. , Candiano, G. , Massazza, S. , Ponzetto, A. , Varesio, L. , New high‐performance liquid chromatographic method for the detection of picolinic acid in biological fluids. J. Chromatogr. B Biomed. Sci. Appl. 2001, 751, 61–68. [DOI] [PubMed] [Google Scholar]
- 84. Heyes, M. , Markey, S. , Quantification of quinolinic acid in rat brain, whole blood, and plasma by gas chromatography and negative chemical ionization mass spectrometry: effects of systemic L‐tryptophan administration on brain and blood quinolinic acid concentrations. Anal. Biochem. 1988, 174, 349–359. [DOI] [PubMed] [Google Scholar]
- 85. Cannazza, G. , Baraldi, M. , Braghiroli, D. , Tait, A. , Parenti, C. , High‐performance liquid chromatographic method for the quantification of anthranilic and 3‐hydroxyanthranilic acid in rat brain dialysate. J. Pharm. Biomed. Anal. 2003, 32, 287–293. [DOI] [PubMed] [Google Scholar]
- 86. Midttun, Ø. , Hustad, S. , Ueland, P. , Quantitative profiling of biomarkers related to B‐vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 1371–1379. [DOI] [PubMed] [Google Scholar]
- 87. Wang, X. D. , Notarangelo, F. M. , Wang, J. Z. , Schwarcz, R. , Kynurenic acid and 3‐hydroxykynurenine production from d‐kynurenine in mice. Brain Res. 2012, 1455, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Luo, X. , Tang, A. , Pi, L. , Ao, X. , Pu, Y. , Wang, R. , Determination of kynurenine in serum by high‐performance liquid chromatography with on‐column fluorescence derivatization. Clin. Chim. Acta 2008, 389, 186–188. [DOI] [PubMed] [Google Scholar]
- 89. Xiao, L. D. , Luo, X. B. , Pi, L. , Tang, A. , Simultaneous determination of kynurenine and kynurenine acid concentrations in human serum by HPLC with dual wavelengths fluorescence detection. Clin. Chim. Acta 2008, 395, 178–180. [DOI] [PubMed] [Google Scholar]
- 90. Holmes, E. , Determination of serum kynurenine and hepatic tryptophan dioxygenase activity by high‐performance liquid chromatography. Anal. Biochem. 1988, 172, 518–525. [DOI] [PubMed] [Google Scholar]
- 91. Vaarmann, A. , Kask, A. , Mäeorg, U. , Novel and sensitive high‐performance liquid chromatographic method based on electrochemical coulometric array detection for simultaneous determination of catecholamines, kynurenine and indole derivatives of tryptophan. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 769, 145–153. [DOI] [PubMed] [Google Scholar]
- 92. Vignau, J. , Jacquemont, M. C. , Lefort, A. , Imbenotte, M. , Lhermitte, M. , Simultaneous determination of tryptophan and kynurenine in serum by HPLC with UV and fluorescence detection. Biomed. Chromatogr. 2004, 18, 872–874. [DOI] [PubMed] [Google Scholar]
- 93. Lan‐Gan, P. , Ai‐Guo, T. , Xi‐Ming, M. , Xi‐Bo, L. , Xiang, A. , More rapid and sensitive method for simultaneous determination of tryptophan and kynurenic acid by HPLC. Clin. Biochem. 2009, 42, 420–425. [DOI] [PubMed] [Google Scholar]
- 94. Hervé, C. , Beyne, P. , Jamault, H. , Delacoux, E. , Determination of tryptophan and its kynurenine pathway metabolites in human serum by high‐performance liquid chromatography with simultaneous ultraviolet and fluorimetric detection. J. Chromatogr. B Biomed. Appl. 1996, 675, 157–161. [DOI] [PubMed] [Google Scholar]
- 95. Pinhati, R. , Polonini, H. , Brandão, M. , Quantification of tryptophan in plasma by high performance liquid chromatography. Quim. Nova 2012, 35, 623–626. [Google Scholar]
- 96. Wang, R. , Tang, A. , Simultaneous determination of kynurenine and tryptophan in serum by high performance liquid chromatography. Chinese J. Chromatogr. 2006, 24, 140–143. [PubMed] [Google Scholar]
- 97. Ma, L. , Xu, B. , Wang, W. , Deng, W. , Ding, M. , Analysis of tryptophan catabolism in HBV patients by HPLC with programmed wavelength ultraviolet detection. Clin. Chim. Acta 2009, 405, 94–96. [DOI] [PubMed] [Google Scholar]
- 98. Zhen, Q. , Xu, B. , Ma, L. , Tian, G. , Tang, X. , Ding, M. , Simultaneous determination of tryptophan, kynurenine and 5‐hydroxytryptamine by HPLC: application in uremic patients undergoing hemodialysis. Clin. Biochem. 2011, 44, 226–230. [DOI] [PubMed] [Google Scholar]
- 99. Amirkhani, A. , Heldin, E. , Markides, K. E. , Bergquist, J. , Quantitation of tryptophan, kynurenine and kynurenic acid in human plasma by capillary liquid chromatography‐electrospray ionization tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 780, 381–387. [DOI] [PubMed] [Google Scholar]
- 100. Xiao, C. , Chen, Y. , Liang, X. , Xie, Z. , Zhang, M. , Li, R. , Li, Z. , Fu, X. , Yu, X. , Shi, W. , A modified HPLC method improves the simultaneous determination of plasma kynurenine and tryptophan concentrations in patients following maintenance hemodialysis. Exp. Ther. Med. 2014, 7, 907–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhang, X. , He, Y. , Ding, M. , Simultaneous determination of tryptophan and kynurenine in plasma samples of children patients with Kawasaki disease by high‐performance liquid chromatography with programmed wavelength ultraviolet detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 1678–1682. [DOI] [PubMed] [Google Scholar]
- 102. Sano, M. , Ferchaud‐Roucher, V. , Nael, C. , Aguesse, A. , Poupeau, G. , Castellano, B. , Darmaun, D. , Simultaneous detection of stable isotope‐labeled and unlabeled L‐tryptophan and of its main metabolites, L‐kynurenine, serotonin and quinolinic acid, by gas chromatography/negative ion chemical ionization mass spectrometry. J. Mass Spectrom. 2014, 49, 128–135. [DOI] [PubMed] [Google Scholar]
- 103. Liu, L. , Chen, Y. , Zhang, Y. , Wang, F. , Chen, Z. , Determination of tryptophan and kynurenine in human plasma by liquid chromatography‐electrochemical detection with multi‐wall carbon nanotube‐modified glassy carbon electrode. Biomed. Chromatogr. 2011, 25, 938–942. [DOI] [PubMed] [Google Scholar]
- 104. Galba, J. , Michalicova, A. , Parrak, V. , Novak, M. , Kovac, A. , Quantitative analysis of phenylalanine, tyrosine, tryptophan and kynurenine in rat model for tauopathies by ultra‐high performance liquid chromatography with fluorescence and mass spectrometry detection. J. Pharm. Biomed. Anal. 2016, 117, 85–90. [DOI] [PubMed] [Google Scholar]
- 105. Laich, A. , Neurauter, G. , Widner, B. ,More rapid method for simultaneous measurement of tryptophan and kynurenine by HPLC. Clin. Chem. 2002, 48, 597–581. [PubMed] [Google Scholar]
- 106. Widner, B. , Werner, E. R. , Schennach, H. , Wachter, H. , Fuchs, D. , Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin. Chem. 1997, 43, 2424–2426. [PubMed] [Google Scholar]
- 107. Hwang, S. L. , Chung, N. P.‐Y. , Chan, J. K.‐Y. , Lin, C.‐L. S. , Indoleamine 2,3‐dioxygenase (IDO) is essential for dendritic cell activation and chemotactic responsiveness to chemokines. Cell Res. 2005, 15, 167–175. [DOI] [PubMed] [Google Scholar]
- 108. Möller, M. , Du Preez, J. , Harvey, B. , Development and validation of a single analytical method for the determination of tryptophan, and its kynurenine metabolites in rat plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 898, 121–129. [DOI] [PubMed] [Google Scholar]
- 109. Baran, H. , Gramer, M. , Hönack, D. , Löscher, W. , Systemic administration of kainate induces marked increases of endogenous kynurenic acid in various brain regions and plasma of rats. Eur. J. Pharmacol. 1995, 286, 167–175. [DOI] [PubMed] [Google Scholar]
- 110. Zagajewski, J. , Drozdowicz, D. , Brzozowska, I. , Hubalewska‐Mazgaj, M. , Stelmaszynska, T. , Laidler, P. M. , Brzozowski, T. , Conversion L‐tryptophan to melatonin in the gastrointestinal tract: the new high performance liquid chromatography method enabling simultaneous determination of six metabolites of L‐tryptophan by native fluorescence and UV‐VIS detection. J. Physiol. Pharmacol. 2012, 63, 613–621. [PubMed] [Google Scholar]
- 111. Zhu, W. , Stevens, A. , Dettmer, K. , Gottfried, E. , Hoves, S. , Kreutz, M. , Holler, E. , Canelas, A. B. , Kema, I. , Oefner, P. J. , Quantitative profiling of tryptophan metabolites in serum, urine, and cell culture supernatants by liquid chromatography‐tandem mass spectrometry. Anal. Bioanal. Chem. 2011, 401, 3249–3261. [DOI] [PubMed] [Google Scholar]
- 112. Wang, H. , McNeil, Y. R. , Yeo, T. W. , Anstey, N. M. , Simultaneous determination of multiple amino acids in plasma in critical illness by high performance liquid chromatography with ultraviolet and fluorescence detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 940, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Krcmova, L. , Solichova, D. , Melichar, B. , Kasparova, M. , Plisek, J. , Sobotka, L. , Solich, P. , Determination of neopterin, kynurenine, tryptophan and creatinine in human serum by high throuput HPLC. Talanta 2011, 85, 1466–1471. [DOI] [PubMed] [Google Scholar]
- 114. Mitsuhashi, S. , Fukushima, T. , Kawai, J. , Tomiy, M. , Santa, T. , Imai, K. , Toyo'oka, T. , Improved method for the determination of kynurenic acid in rat plasma by column‐switching HPLC with post‐column fluorescence detection. Anal. Chim. Acta 2006, 562, 36–43. [Google Scholar]
- 115. Mitsuhashi, S. , Fukushima, T. , Tomiya, M. , Santa, T. , Imai, K. , Toyo'oka, T. , Determination of kynurenine levels in rat plasma by high‐performance liquid chromatography with pre‐column fluorescence derivatization. Anal. Chima Acta 2007, 584, 315–321. [DOI] [PubMed] [Google Scholar]
- 116. Mitsuhashi, S. , Fukushima, T. , Arai, K. , Tomiya, M. , Santa, T. , Imai, K. , Toyo`oka, T. , Development of a column‐switching high‐performance liquid chromatography for kynurenine enantiomers and its application to a pharmacokinetic study in rat plasma. Anal. Chim. Acta 2007, 587, 60–66. [DOI] [PubMed] [Google Scholar]
- 117. Fukushima, T. , Mitsuhashi, S. , Tomiya, M. , Iyo, M. , Hashimoto, K. , Toyo'oka, T. , Determination of kynurenic acid in human serum and its correlation with the concentration of certain amino acids. Clin. Chim. Acta 2007, 377, 174–178. [DOI] [PubMed] [Google Scholar]
- 118. Fukushima, T. , Mitsuhashi, S. , Tomiya, M. , Kawai, J. , Hashimoto, K. , Toyo'oka, T. , Determination of rat brain kynurenic acid by column‐switching HPLC with fluorescence detection. Biomed. Chromatogr. 2007, 21, 514–519. [DOI] [PubMed] [Google Scholar]
- 119. Notarangelo, F. , Wu, H. , Macherone, A. , Graham, D. , Schwarcz, R. , Gas chromatography/tandem mass spectrometry detection of extracellular kynurenine and related metabolites in normal and lesioned rat brain. Anal. Biochem. 2012, 421, 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Takahashi, S. , Iizuka, H. , Kuwabara, R. , Naito, Y. , Sakamoto, T. , Miyagi, A. , Onozato, M. , Ichiba, H. , Fukushima, T. , Determination of l‐tryptophan and l‐kynurenine derivatized with (R)‐4‐(3‐isothiocyanatopyrrolidin‐1‐yl)‐7‐(N,N‐dimethylaminosulfonyl)‐2,1, 3‐benzoxadiazole by LC‐MS/MS on a triazole‐bonded column and their quantification in human serum. Biomed. Chromatogr. 2016, 30, 1481–1486. [DOI] [PubMed] [Google Scholar]
- 121. Yamada, K. , Miyazzaki, T. , Shibata, T. , Hara, N. , Tsuchiya, M. , Simultaneous measurement of tryptophan and related compounds by liquid chromatography/electrospray ionization tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 867, 57–61. [DOI] [PubMed] [Google Scholar]
- 122. Sultana, N. , Arayne, M. S. , Khan, M. M. , Saleem, D. M. , Mirza, A. Z. , Determination of tryptophan in raw materials, rat brain and human plasma by RP‐HPLC technique. J. Chromatogr. Sci. 2012, 50, 531–537. [DOI] [PubMed] [Google Scholar]
- 123. Marklová, E. , Makovičková, H. , Krákorová, I. , Screening for defects in tryptophan metabolism. J. Chromatogr. A 2000, 870, 289–293. [DOI] [PubMed] [Google Scholar]
- 124. Ohashi, H. , Iizuka, H. , Yoshihara, S. , Otani, H. , Iizuka, H. , Yoshihara, S. , Otani, H. , Kume, M. , Sadamoto, K. , Ichiba, H. , Fukushima, T. , Determination of l‐tryptophan and l‐kynurenine in human serum by using LC‐MS after derivatization with (R)‐DBD‐PyNCS. Int. J. Tryptophan Res. 2013, 6, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Iizuka, H. , Ishii, K. , Hirasa, Y. , Kubo, K. , Fukushima, T. , Fluorescence determination of d‐ and l‐tryptophan concentrations in rat plasma following administration of tryptophan enantiomers using HPLC with pre‐column derivatization. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 3208–3213. [DOI] [PubMed] [Google Scholar]
- 126. Dobbie, M. S. , Surtees, R. A. H. , Concentrations of quinolinic acid in cerebrospinal fluid measured by gas chromatography and electron‐impact ionisation mass spectrometry: age‐related changes in a paediatric reference population. J. Chromatogr. B Biomed. Sci. Appl. 1997, 696, 53–58. [DOI] [PubMed] [Google Scholar]
- 127. Naritsin, D. B. , Boni, R. L. , Markey, S. P. , Pentafluorobenzylation method for quantification of acidic tryptphan metabolites using electron capture negative ion mass spectrometry. Anal. Chem. 1995, 67, 863–870. [DOI] [PubMed] [Google Scholar]
- 128. Huang, Y. , Louie, A. , Yang, Q. , Massenkoff, N. , Xu, C. , Hunt, P. W. , Gee, W. , A simple LC‐MS/MS method for determination of kynurenine and tryptophan concentrations in human plasma from HIV‐infected patients. Bioanal 2013, 5, 1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Meinitzer, A. , Tomaschtz, A. , Pilz, S. , Truber, M. , Zechner, G. , Gaksch, M. , Prietl, B. , Schwarz, M. , Baranyi, A. , Development of a liquid chromatography‐mass spectrometry method for the determination of the neurotoxic quinolinic acid in human serum. Clin. Chim. Acta 2014, 436, 268–272. [DOI] [PubMed] [Google Scholar]
- 130. de Jong, W. H. A. , Smit, R. , Bakker, S. J. L. , de Vries, E. G. E. , Kema, I. P. , Plasma tryptophan, kynurenine and 3‐hydroxykynurenine measurement using automated on‐line solid‐phase extraction HPLC‐tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 603–609. [DOI] [PubMed] [Google Scholar]
- 131. Stokvis, E. , Rosing, H. , Beijnen, J. , Stable isotopically labeled internal standards in quantitative bioanalysis using liquid chromatography/mass spectrometry: necessity or not? Rapid Commun. Mass Spectrom. 2005, 19, 401–407. [DOI] [PubMed] [Google Scholar]
- 132. Carlá, V. , Lombardi, G. , Beni, M. , Russi, P. , Moneti, G. , Moroni, F. , Identification and measurement of kynurenic acid in the rat brain and other organs. Anal. Biochem. 1988, 169, 89–94. [DOI] [PubMed] [Google Scholar]
- 133. Kema, I. P. , Schellings, A. M. J. , Hoppenbrouwers, C. J. M. , Rutgers, H. M. , de Vries, E. G. E. , Muskiet, F. A. J. , High performance liquid chromatographic profiling of tryptophan and related indoles in body fluids and tissues of carcinoid patients. Clin. Chim. Acta 1993, 221, 143–158. [DOI] [PubMed] [Google Scholar]
- 134. Kawai, K. , Ishikawa, H. , Ohashi, K. , Itoh, Y. , Teradaira, R. , Rapid, simple and simultaneous measurement of kynurenine and tryptophan in plasma by column switching‐HPLC method. Int. Congr. Ser. 2007, 1304, 415–419. [Google Scholar]
- 135. Bizzarri, M. , Catizone, A. , Determination of urinary tryptophan and its metabolites along the nicotinic acid pathway by high performance liquid chromatography with ultraviolet detection. Biomed. Chromatogr. 1990, 4, 24–27. [DOI] [PubMed] [Google Scholar]
- 136. Krcmova, L. , Cervinkova, B. , Solichova, D. , Sobotka, L. , Hansmanova, L. , Melichar, B. , Solich, P. , Fast and sensitive HPLC method for the determination of neopterin, kynurenine and tryptophan in amniotic fluid, malignant effusions and wound exudates. Bioanal 2015, 7, 2751–2762. [DOI] [PubMed] [Google Scholar]
- 137. Li, Y. , Tang, A.‐G. , Mu, S. , HPLC‐FLD determination of serum aromatic amino acids: application in chronic kidney disease patients. Clin. Chim. Acta 2011, 412, 1032–1035. [DOI] [PubMed] [Google Scholar]
- 138. Maneglier, B. , Rogez‐Kreuz, C. , Cordonnier, P. , Therond, P. , Advenier, C. , Dormont, D. , Clayette, P. , Spreux‐Varoquaux, O. , Simultaneous measurement of kynurenine and tryptophan in human plasma and supernatants of cultured human cells by HPLC with coulometric detection. Clin. Chem. 2004, 50, 2166–2168. [DOI] [PubMed] [Google Scholar]
- 139. Arvidsson, B. , Johannesson, N. , Citterio, A. , Righetti, P. , Bergquist, J. , High throughput analysis of tryptophan metabolites in a complex matrix using capillary electrophoresis coupled to time‐of‐flight mass spectrometry. J. Chromatogr. A 2007, 1159, 154–158. [DOI] [PubMed] [Google Scholar]
- 140. Lu, H. , Yu, J. , Wang, J. , Wu, L. , Xiao, H. , Gao, R. , Simultaneous quantification of neuroactive dopamine serotonin and kynurenine pathway metabolites in gender‐specific youth urine by ultra performance liquid chromatography tandem high resolution mass spectrometry. J. Pharm. Biomed. Anal. 2016, 122, 42–51. [DOI] [PubMed] [Google Scholar]
- 141. Zhang, L.‐H. , Cai, H.‐L. , Jiang, P. , Li, H.‐D. , Cao, L.‐J. , Dang, R.‐L. , Zhu, W.‐Y. , Deng, Y. , Simultaneous determination of multiple neurotransmitters and their metabolites in rat brain homogenates and microdialysates by LC‐MS/MS. Anal. Methods 2015, 7, 3929–3938. [Google Scholar]
- 142. Hayashi, T. , Shimamura, M. , Matsuda, F. , Minatogawa, Y. , Naruse, H. , Iida, Y. , Sensitive determination of deuterated and non‐deuterated tryptophan, tryptamine and serotonin by combined capillary gas chromatography and negative ion chemical ionization mass spectrometry. J. Chromatogr. 1986, 383, 259–269. [DOI] [PubMed] [Google Scholar]
- 143. Wang, W. , Qiu, B. , Xu, X. , Zhang, L. , Chen, G. , Separation and determination of L‐tryptophan and its metabolites by capillary micellar electrokinetic chromatography with amperometric detection. Electrophoresis 2004, 25, 903–910. [DOI] [PubMed] [Google Scholar]
- 144. Mawatari, K. , Iinuma, F. , Watanabe, M. , Fluorimetric determination of kynurenine in human serum by high‐performance liquid chromatography coupled with post‐column photochemical reaction with hydrogen peroxide. J. Chromatogr. 1989, 488, 349–355. [DOI] [PubMed] [Google Scholar]
- 145. Iizuka, H. , Hirasa, Y. , Kubo, K. , Ishii, K. , Toyo'oka, T. , Fukushima, T. , Enantiomeric separation of D,L‐tryptophan and D,L‐kynurenine by HPLC using pre‐column fluorescence derivatization with R(‐)‐DBD‐PyNCS. Biomed. Chromatogr. 2011, 25, 743–747. [DOI] [PubMed] [Google Scholar]
- 146. Iwasaki, M. , Kashiwaguma, Y. , Nagashima, C. , Izumia, M. , Uekusa, A. , Iwasa, S. , Onozato, M. , Ichiba, H. , Fukushima, T. , A high‐performance liquid chromatography assay with a triazole‐bonded column for evaluation of d‐amino acid oxidase activity. Biomed. Chromatogr. 2016, 30, 384–389. [DOI] [PubMed] [Google Scholar]
- 147. Kozaki, A. , Iwasa, S. , Hosoda, S. , Nishiguchi, Y. , Nakayama, M. , Ichiba, H. , Fukushima, T. , Fluorimetric assay for D‐amino acid oxidase activity in rat brain homogenate by using D‐kynurenine as a substrate. Biosci. Trends 2012, 6, 241–247. [DOI] [PubMed] [Google Scholar]
- 148. Morita, I. , Kawamoto, M. , Hattori, M. , Eguchi, K. , Sekiba, K. , Yoshida, H. , Determination of tryptophan and its metabolites in human plasma and serum by high‐performance liquid chromatography with automated sample clean‐up system. J. Chromatogr. 1990, 526, 367–374. [DOI] [PubMed] [Google Scholar]
- 149. Swartz, K. , Matson, W. , MacGarvey, U. , Ryan, E. , Beal, M. , Measurement of kynurenic acid in mammalian brain extracts and cerebrospinal fluid by high‐performance liquid chromatography with fluorometric and coulometric electrode array detection. Anal. Biochem. 1990, 185, 363–376. [DOI] [PubMed] [Google Scholar]
- 150. Swartz, K. J. , During, M. J. , Freese, A. , Beal, M. F. , Cerebral synthesis and release of kynurenic acid: an endogenous antagonist of excitatory amino acid receptors. J. Neurosci. 1990, 10, 2965–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Heyes, M. , Quearry, B. , Quantification of kynurenic acid in cerebrospinal fluid: effects of systemic and central L‐kynurenine administration. J. Chromatogr. 1990, 530, 108–115. [DOI] [PubMed] [Google Scholar]
- 152. Lesniak, W. G. , Jyoti, A. , Mishra, M. K. , Louissaint, N. , Romero, R. , Chugani, D. C. , Kannan, S. , Kannan, R. M. , Concurrent quantification of tryptophan and its major metabolites. Anal. Biochem. 2013, 443, 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Andrzejewska‐Buczko, J. , Pawlak, D. , Tankiewicz, A. , Matys, T. , Buczko, W. , Possible involvement of kynurenamines in the pathogenesis of cataract in diabetic patients. Med. Sci. Monit. 2001, 7, 742–745. [PubMed] [Google Scholar]
- 154. Shibata, K. , Fluorimetric micro‐determination of kynurenic acid, an endogenous blocker of neurotoxicity, by high‐performance liquid chromatography. J. Chromatogr. 1988, 430, 379–380. [DOI] [PubMed] [Google Scholar]
- 155. Li, J. , Li, G. , Identification of 3‐hydroxykynurenine and xanthurenic acid and quantitation of 3‐hydroxykynurenine transaminase activity using HPLC with electrochemical detection. J. Liq. Chrom. Rel. Technol. 1998, 21, 1511–1525. [Google Scholar]
- 156. Shibata, K. , Onodera, M. , High‐performance liquid chromatographic determination of 3‐hydroxykynurenine with fluorimetric detection; comparison of preovulatory phase and postovulatory phase urinary excretion. J. Chromatogr. B Biomed. Sci. Appl. 1991, 570, 13–18. [DOI] [PubMed] [Google Scholar]
- 157. Fornstedt‐Wallin, B. , Lundström, J. , Fredriksson, G. , Schwarcz, R. , Luthman, J. , 3‐Hydroxyanthranilic acid accumulation following administration of the 3‐hydroxyanthranilic acid 3,4‐dioxygenase inhibitor NCR‐631. Eur. J. Pharmacol. 1999, 386, 15–24. [DOI] [PubMed] [Google Scholar]
- 158. Ubbink, J. B. , Vermaak, W. J. , Bissbort, S. H. , High‐performance liquid chromatographic assay of human lymphocyte kynureninase activity levels. J. Chromatogr. 1991, 566, 369–375. [DOI] [PubMed] [Google Scholar]
- 159. Heyes, M. P. , Achim, C. L. , Wiley, C. A. , Major, E. O. , Saito, K. , Markey, S. P. , Human microglia convert l‐tryptophan into the neurotoxin quinolinic acid. Biochem. J. 1996, 320, 595–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Eckstein, J. A. , Ammerman, G. M. , Reveles, J. M. , Ackermann, B. L. , Simultaneous profiling of multiple neurochemical pathways from a single cerebrospinal fluid sample using GC–MS/MS with electron capture detection. J. Mass Spectrom. 2008, 43, 782–790. [DOI] [PubMed] [Google Scholar]
- 161. Watson, E. , Wilk, S. , Derivatization and gas chromatographic determination of some biologically important acids in cerebrospinal fluid. Anal. Biochem. 1974, 59, 441–451. [DOI] [PubMed] [Google Scholar]
- 162. Smythe, G. , Braga, O. , Brew, B. , Grant, R. , Guillemin, G. J. , Kerr, S. J. , Walker, D. W. , Concurrent quantification of quinolinic, picolinic, and nicotinic acids using electron‐capture negative‐ion gas chromatography‐mass spectrometry. Anal. Biochem. 2002, 301, 21–26. [DOI] [PubMed] [Google Scholar]
- 163. Dobbie, M. S. , Surtees, R. A. , Concentrations of quinolinic acid in cerebrospinal fluid measured by gas chromatography and electron‐impact ionisation mass spectrometry: age‐related changes in a paediatric reference population. J. Chromatogr. B Biomed. Sci. Appl. 1997, 696, 53–58. [DOI] [PubMed] [Google Scholar]
- 164. Joseph, M. H. , Baker, H. F. , Crow, T. J. , Riley, G. J. , Risby, D. , Brain tryptophan metabolism in schizophrenia: a post mortem study of metabolites on the serotonin and kynurenine pathways in schizophrenic and control subjects. Psychopharmacology 1979, 62, 279–285. [DOI] [PubMed] [Google Scholar]


