Table 4.
Global Deprotection to the Target α-(2′Z-Fluoro)vinyl-AA’sa
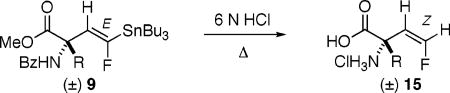
Procedure: The fully protected stannylvinyl amino acid (9) was suspended in 6 N HCl and refluxed for 12–30 h.
R groups are given as they are found in the educts 9. Under the reaction conditions, the side chain of aspartate is de-esterified, that of lysine is debenzoylated, and those of m-Tyr and DOPA are desilylated. Geometry is assigned as Z as J1,2(H–F) = 43–46 Hz, as opposed to ≈17 Hz for E, see ref 10].
These fluorovinyl AA’s were isolated as their hydrochloride salts.
Further purified by Dowex 50 cation exchange chromatography.