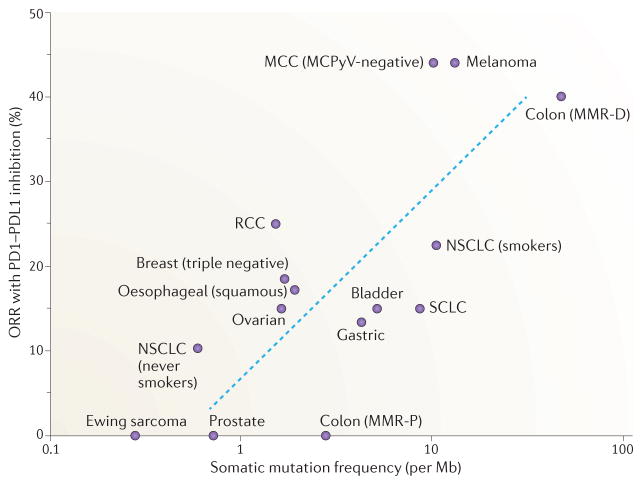
Figure 2. Correlation of tumour somatic mutation frequency with objective response rates to immune checkpoint blockade.
The full potential of programmed cell death protein 1 (PD1)–PD1 ligand 1 (PDL1) checkpoint inhibitors remains undefined; however, from the available clinical data there is a positive correlation between tumour somatic mutation frequency and clinical benefit, measured by the objective response rate (ORR). The ORR is defined in the context of these clinical studies as the proportion of patients who achieved 30% decrease in the sum of the longest diameters of target tumours based on modified Response Evaluation Criteria in Solid Tumours (RECIST)157. Here, we show the somatic mutation frequency (somatic mutations per megabase) and ORR to single-agent PD1 or PDL1 inhibition across multiple solid tumours of non-viral origin for which clinical and sequencing data are available. Tumours with the highest somatic mutation rates (mismatch repair-deficient (MMR-D) colon cancer, Merkel cell polyomavirus (MCPyV)-negative Merkel cell carcinoma (MCC), melanoma and non-small-cell lung cancer (NSCLC) in current or prior tobacco smokers) have amongst the highest ORRs to PD1 PDL1 inhibition (23–44%), whereas tumours with lower mutation rates have demonstrated less frequent responses. These data are obtained from a diverse group of clinical trials conducted at different stages of disease and included are some early-stage exploratory clinical trials, which probably accounts for some of the variability that is observed. However, extrapolating from these data, we can predict the ORR to single-agent PD1–PDL1 blockade for any tumour type with the formula ORR (%) = 0.08 × ln(x)+ 9, where x is the somatic mutation frequency per Mb of DNA. The blue dashed line on the graph represents the line of best fit calculated from the formula. Many PD1–PDL1 inhibitor trials are ongoing and will further clarify the slope of this correlation. MMR-P, mismatch repair proficient; RCC, renal cell carcinoma; SCLC, small-cell lung cancer.