Abstract
Background
Tuberculosis (TB) remains a major challenge to global health. Healthcare workers (HCWs) appear to be at increased risk of TB compared with the general population, despite efforts to scale up infection control and reduce nosocomial TB transmission. This review aims to provide an updated estimate of the occupational risk of latent TB infection (LTBI) and active TB among HCWs compared with the general population.
Methods
A systematic review was performed to identify studies published over the last 10 years reporting TB prevalence or incidence among HCWs and a control group. Pooled effect estimates were calculated to determine the risk of infection.
Results
Twenty-one studies met the inclusion criteria, providing data on 30961 HCWs across 16 countries. Prevalence of LTBI among HCWs was 37%, and mean incidence rate of active TB was 97/100000 per year. Compared with the general population, the risk of LTBI was greater for HCWs (odds ratio [OR], 2.27; 95% confidence interval [CI], 1.61–3.20), and the incidence rate ratio for active TB was 2.94 (95% CI, 1.67–5.19). Comparing tuberculin skin test and interferon-gamma release assay, OR for LTBI was found to be 1.72 and 5.61, respectively.
Conclusions
The overall risk of both LTBI and TB to HCWs continues to be significantly higher than that of the general population, consistent with previous findings. This study highlights the continuing need for improvements in infection control and HCW screening programs.
Keywords: healthcare workers, incidence, occupational risk, prevalence, tuberculosis
According to the World Health Organization (WHO), there were 10.4 million new cases of tuberculosis (TB) in 2015 and 1.4 million deaths [1], representing a significant challenge to global health. India, Indonesia, China, Nigeria, Pakistan, and South Africa accounted for 60% of incident cases, suggesting that further reduction in TB cases is likely dependent on improved prevention and care in these countries to reduce the considerable gap between number of incident cases and those that are identified and treated appropriately [1].
To combat the epidemic, the WHO introduced their “END TB” Strategy in 2015, which aims to reduce TB incidence by 95% by 2030 [2], and infection control was included as a key component of this strategy. This is particularly important for healthcare workers (HCWs) who, through nosocomial transmission, are likely to have increased exposure to TB and therefore are at greater risk of contributing to TB transmission.
Healthcare workers are known to be at high risk of latent TB infection (LTBI) and active TB disease through occupational exposure to patients with active TB [3], and pathogen sequencing is now able to track transmission in healthcare settings [4, 5]. Although this has been explored in previous reviews, there is a need to update estimates in light of changing TB prevalence and infection control policies. Previous reviews have covered large periods of time [6–8], and since then TB treatment and control has greatly improved. This outcome is likely to have had a significant impact on their results. Our study aims to review the current TB risk to HCWs, which is particularly pertinent because it occurs at the beginning of the Sustainable Development Goals and the WHO’s END TB strategy. The primary aims of this review and meta-analysis are to (1) provide an updated estimate of the occupational risk of LTBI and active TB to HCWs compared with the general population and (2) to compare the incidence or prevalence between the 2 groups.
METHODS
This meta-analysis was reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9].
Search Strategy
Electronic searches were performed using Ovid in MEDLINE, Embase, and Global Health up to March 23, 2016. Studies published before January 1, 2006 were excluded due to their comprehensive coverage in earlier reviews and because our focus was on more recent estimates. The search strategy is described in detail in Supplementary Appendix.
Healthcare workers were first defined broadly according to the WHO definition of “people engaged in the promotion, protection or improvement of the health of the population” [10] and then more specifically as those used in healthcare and in direct contact with patients. Control groups included (1) administrative HCWs who were not in direct contact with patients as well as (2) comparable groups of non-HCWs. Studies that used national data to calculate population prevalence or incidence were included as long as the method of calculation was clear and the comparison was appropriate. Studies that used reference data for the general population were excluded if their estimates were taken from other studies and it was not clear how this had been calculated, meaning the comparison could not be guaranteed to be appropriate.
Observational studies included cohort and case-control studies, including cross-sectional studies. Conference abstracts were excluded due to an inability to extract the relevant data and to assess methodological quality. Reviews and case reports were excluded.
Comorbidities such as human immunodeficiency virus (HIV), chronic kidney disease, and diabetes were excluded if the primary aim of the study was to compare TB in these groups to populations without comorbidity, because of the well known increased risk of infection in these populations [11–13]. However, if populations of HCWs and non-HCWs were later found to include these groups, they were not excluded from the analysis. The initial screen had no language limitations, but the final full-text screen only included studies published in English. No geographic limitations were applied.
Attempts were made to include studies looking at both incidence and prevalence of both LTBI and active TB disease. After we screened the initial papers, it became apparent that those using incidence had focused on active TB disease, whereas those using prevalence focused on LTBI, and so these were analyzed separately to produce a more reliable comparison. This means that, throughout this paper, references to prevalence refer to LTBI, whereas incidence refers to active disease. Prevalence of LTBI was either stated in papers as a primary outcome or an assumption was made by using tuberculin skin test (TST) or interferon-gamma release assay (IGRA) (mainly TST >10 mm or 5 mm in those who were HIV positive). Studies that used TB notification rates were assumed to be referring to incidence rates (IRs) of active TB; however, a lack of a clear positive definition was highlighted in the score the paper received in the quality assessment. If both TST and IGRA were used in a paper, TST was used to analyze prevalence because this was the more commonly used test and therefore allowed investigators to obtain a more reliable comparison.
The following information was extracted from all studies according to a predetermined data extraction form: title, date of publication, author, country of study, language of study, funding source, study design, length of study, diagnostic method, type of HCW, type of control group, and whether the study assessed incidence of active TB or prevalence of LTBI.
For studies investigating prevalence of LTBI, the number of HCWs and controls and the number of cases (positive TST or IGRA) in both HCWs and controls were recorded. If studies implemented multiple testing methods, then only the initial results were used because later screening may have biased results due to increased awareness of occupational TB risk.
For studies investigating incidence of active disease, the number of cases (diagnosis of TB by various methods) and person-years (py) among HCWs were recorded, if available. If only cases and IR were given, these were used to calculate the py of the study, and if the IR for multiple years had been recorded for different years of the study, the mean of these was calculated. For the control groups only, IR per 100000 per annum was available for all studies; therefore, this denominator was used for all control groups.
Quality Assessment
Methodological quality of studies was assessed using items of the STROBE checklist [14], and, using this approach, studies were ranked into high (>55), medium (≤55), and low (≤45) quality. Although all studies were included in the original meta-analysis, subgroup analysis was later done excluding low-quality studies.
Statistical Methods
Meta-Analysis
From the data extracted from each study, using total number of cases among HCWs and total number of HCW participants, a pooled prevalence estimate of LTBI among HCWs was calculated. The Mantel-Haenszel (MH) method for dichotomous outcomes was then used to calculate odds ratios (ORs). For studies investigating incidence of active TB, the MH method was used to calculate IR ratios (IRRs). Ninety-five percent confidence intervals (CIs) were generated for all estimates. If a study included data for both incidence of active TB and prevalence of LTBI, it was included in both meta-analyses. All meta-analyses were conducted using random-effects models.
To investigate possible causes of heterogeneity, subgroup analysis was performed. First, low-quality studies were excluded. Then, from the remaining studies, analyses were performed by TB burden and TB/HIV coinfection burden according to WHO-defined groups [15], method of diagnosis, and income group according to World Bank definitions [16]. An additional subgroup analysis was then performed, which included only the incidence studies that either reported py for the control groups or reported incidence for which py could be calculated. All analyses were carried out using R.
RESULTS
Study Characteristics
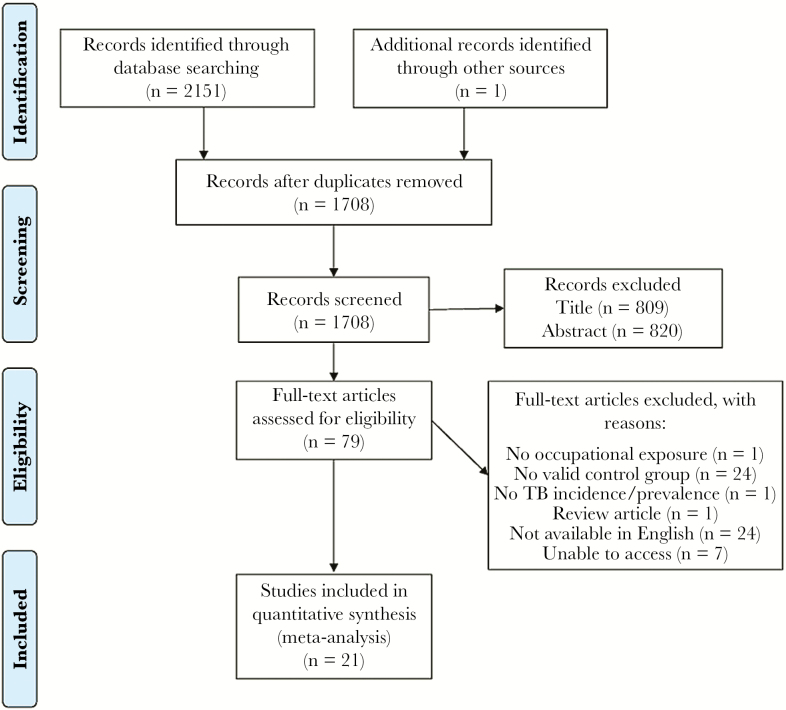
From an initial screen of 2152 publications, 21 met the inclusion criteria and were included in the meta-analysis (Figure 1). Twelve studies investigated prevalence of LTBI among HCWs (9 cross-sectional studies, 3 cohort studies), 8 investigated incidence of active TB (7 cohort studies and 1 cross-sectional study), and 1 cohort study compared both. Only 1 study included a matched control group [17], whereas the others were unmatched. A total of 8 studies were included from Asia, 5 from Africa, 5 from Europe, and 3 from South America. Four studies investigated HCWs with high exposure to TB, whereas the remainder looked at HCWs in general. The control groups included school workers, nonmedical students, administrative employees, and reference data for the general population. Study characteristics are summarized in Tables 1 and 2.
Figure 1.
Flow diagram illustrating literature search and study selection. Abbreviation: TB, tuberculosis.
Table 1.
Characteristics of Included Studies Reporting Prevalence
| Author | Country | WHO HBC | High TB/HIV Burden | Income Group | Study Design | Study Size | HCWs | No. of HCWs | Controls | No. of Controls | Method of Dx | No. of Cases/ HCW | Prevalence among HCWs (%) | Odds Ratio | Study Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agaya et al [18] | Kenya | Yes | Yes | Lower-Middle | Cross-sectional | 1230 | G | 898 | SW | 332 | TST | 534 | 59.5 | 1.58 (1.22; 2.03) | HIGH(56) |
| Corbett et al [3] | Zimbabwe | Yes | Yes | Low | Cohort | 785 | S | 342 | NS | 443 | TST | 183 | 54.0 | 0.90 (0.68; 1.20) | HIGH(57) |
| Drobniewski et al [19] | Russia | Yes | Yes | High | Cross-sectional | 392 | G | 262 | NS | 130 | IGRA | 107 | 40.8 | 10.53 (4.94; 22.43) | HIGH(57) |
| Durando et al [20] | Italy | No | No | High | Cross-sectional | 721 | S | 585 | NS | 136 | TST | 3 | 0.5 | 0.23 (0.05; 1.14) | MEDIUM(52) |
| Franco and Zanetta [21] | Brazil | Yes | Yes | Upper-Middle | Cross-sectional | 333 | H | 169 | A | 164 | TST | 101 | 59.8 | 1.28 (0.83; 1.98) | MEDIUM(54) |
| Maciel et al [22] | Brazil | Yes | Yes | Upper-Middle | Cohort | 837 | S | 619 | NS | 218 | TST | 114 | 18.4 | 3.88 (2.09; 7.18) | LOW(43) |
| Sawhney et al [23] | India | Yes | Yes | Lower-Middle | Cohort | 200 | G | 100 | A | 100 | TST | 20 | 20.0 | 2.53 (1.09; 5.87) | LOW(41) |
| Nikokar et al [24] | Iran | No | No | Upper-Middle | Cross-sectional | 366 | G | 185 | SW | 181 | TST | 113 | 61.1 | 2.03 (1.34; 3.070) | LOW(35) |
| Powell et al [25] | Vietnam | Yes | Yes | Lower-Middle | Cross-sectional | 1111 | G | 956 | SW | 155 | TST | 380 | 39.7 | 1.90 (1.29; 2.78) | HIGH(58) |
| Rutanga et al [26] | Rwanda | No | Yes | Low | Cross-sectional | 1371 | G | 1023 | SW | 348 | TST | 635 | 62.1 | 2.58 (2.01; 3.32) | HIGH(59) |
| Storla et al [27] | Norway | No | No | High | Cohort | 203 | H | 155 | A | 48 | TST | 42 | 27.1 | 5.58 (1.64; 18.91) | MEDIUM(46) |
| Zhu et al [28] | China | Yes | Yes | Upper-Middle | Cross-sectional | 105 | G | 20 | R | 85 | IGRA | 6 | 30.0 | 4.78 (1.40: 16.34) | MEDIUM(54) |
| van Rie et al [29] | South Africa | Yes | Yes | Upper-Middle | Cross-sectional | 199 | H | 120 | NS | 79 | TST | 68 | 56.7 | 3.61 (1.95; 6.69) | MEDIUM(54) |
Abbreviations: A, administrative employee; Dx, diagnosis; G, general healthcare workers; H, healthcare workers working specifically with TB; HBC, high-burden country; HCWs, healthcare workers; HIV, human immunodeficiency virus; IGRA, interferon-gamma release assay; NS, nonclinical students; R, reference data for wider population; S, medical or nursing students; SW, school workers; TB, tuberculosis; TST, tuberculin skin test; WHO, World Health Organization.
Table 2.
Characteristics of Included Studies Reporting Incidence
| Author | Country | WHO HBC | High TB/HIV Burden | Income Group | Study Design | HCWs | Person-Years/ HCWs | Controls | Method of Dx | No. of Cases/ HCW | Incidence Among HCWs/100000 per Annum | IRR | Study Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al [30] | China | Yes | Yes | Upper-Middle | Cohort | G | 181 724 | R | N | 142 | 78.3 | 1.91 (1.35; 2.70) | MEDIUM(48) |
| Chu et al [17] | Taiwan | No | No | High | Cohort | G | 101 505 | R | Rx | 62 | 61.08 | 1.65 (1.10; 2.48) | HIGH(56) |
| Claassens et al [31] | South Africa | Yes | Yes | Upper-Middle | Cohort | HR | 250 | R | Rx | 11 | 4393 | 2.35 (1.30; 4.24) | MEDIUM(52) |
| Corbett et al [3] | Zimbabwe | Yes | Yes | Low | Cohort | S | 155 | NS | T | 30 | 19 300 | 3.23 (2.25; 4.62) | HIGH(57) |
| Klimuk et al [32] | Belarus | No | No | Upper-Middle | Cohort | H | 5445 | R | N | 23 | 426 | 9.82 (5.92; 16.30) | LOW(39) |
| Pan et al [33] | Taiwan | No | No | High | Cohort | G | 4980 | R | C | 3 | 63.1 | 1.83 (0.56; 5.95) | MEDIUM(54) |
| Pazin-Filho et al [34] | Brazil | Yes | Yes | Upper-Middle | Cohort | G | 4520 | R | C | 5 | 110.6 | 2.63 (1.04; 6.66) | LOW (24) |
| Skodric-Trifuno et al [35] | Serbia | No | No | Upper-Middle | Cohort | G | 57 279 | R | C | 24 | 41.9 | 1.23 (0.73; 2.08) | MEDIUM(49) |
| Sotgiu et al [36] | Romania | No | No | Upper-Middle | Cross-sectional | G | 5303 | R | C | 50 | 942.8 | 9.72 (6.91; 13.67) | LOW(42) |
Abbreviations: C, culture positive; Dx, diagnosis; G, general healthcare workers; H, healthcare workers working specifically with TB; HBC, high-burden country; HCWs, healthcare workers; HIV, human immunodeficiency virus; HR, healthcare researchers; IRR, incidence rate ratio; N, not given; NS, nonclinical students; R, reference data for wider population; Rx, recorded treatment; S, medical or nursing students; TB, tuberculosis; WHO, World Health Organization.
Quality Assessment and Within Study Bias
Most papers had clear inclusion criteria for HCWs and control groups, thereby reducing selection bias; however, the objectivity of diagnostic methods used varied between studies, with some having clear definitions of a positive result. Nonetheless, others failed to define which exact method was used to determine a positive result. Withdrawal rates also varied between studies and did introduce an element of attrition bias to some reports.
There was considerable heterogeneity between studies, and many studies included small sample sizes, as demonstrated by the wide and overlapping CIs generated. Therefore, a funnel plot to assess publication bias was deemed to be unreliable.
Prevalence of Latent Tuberculosis Infection
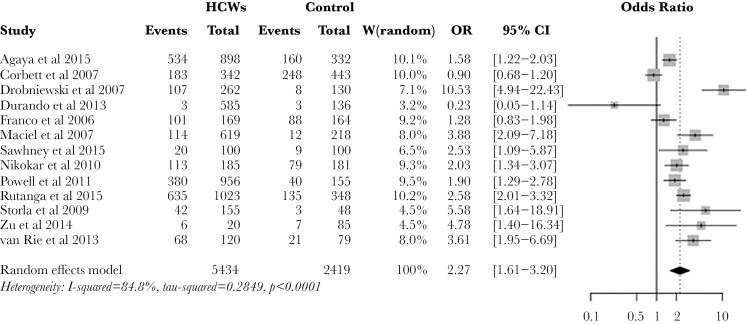
The pooled prevalence estimate for LTBI among HCWs was 37% (95% CI, 28%–47%), with 6 studies reporting prevalence of more than 50%, although estimates ranged from 0.5% to 62%. The lowest prevalence rates were seen in 2 of the 3 studies that compared medical or nursing students to the general population. Of the 3 studies comparing HCWs with an especially high likelihood of TB exposure, the prevalence was towards the higher end of the range, except for the 1 study that was carried out in a high-income country (HIC). The OR for LTBI in HCWs was 2.27 (95% CI, 1.61–3.20) (Figure 2), compared with the general population.
Figure 2.
Forest plot showing pooled odds ratio (OR) for latent tuberculosis infection among healthcare workers (HCWs). Abbreviation: CI, confidence interval.
Subgroup analyses were carried out, which were restricted to high- and medium-quality studies. When the analysis was restricted to high-burden countries (HBCs), the OR for LTBI was 2.23 (95% CI, 1.37–3.62), compared with 1.74 (95% CI, 0.46–6.54) for countries without a high TB burden. The risk of LTBI was higher in countries with a high TB/HIV coinfection burden (OR, 2.25; 95% CI, 1.48–3.43), compared with countries without a high TB/HIV coinfection burden (OR, 1.18; 95% CI, 0.05–28.9).
The risk of TB infection was lower in studies that used TST (OR, 1.72; 95% CI, 1.17–2.52) compared with studies that used IGRA (OR, 5.61; 95% CI, 3.19–9.89). This difference was explored by comparing the prevalence estimates in both the HCW and control groups. The TST gave a pooled prevalence estimate of 37% (95% CI, 27%–49%) for HCWs and 24% (95% CI, 17%–35%) for the control groups, whereas IGRA gave results of 28% (95% CI, 10%–57%) and 8% (95% CI, 4.4%–15%), respectively, suggesting that the difference in OR arises mainly from a reduced number of cases among the control groups rather than among HCWs.
Incidence of Active Tuberculosis
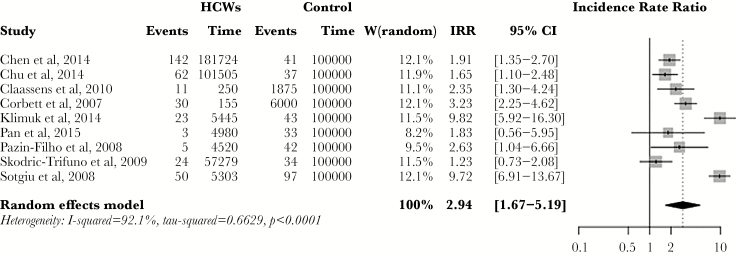
The pooled estimate for incidence of active TB among HCWs was 97/100000 py (range, 42 to 4393/1000000 py), whereas the IRR for active TB among HCWs compared with the general population was 2.94 (95% CI, 1.67–5.19) (Figure 3). When low-quality studies were excluded, the IRR was 1.99 (95% CI, 1.47–2.69). Restricting the analysis to low- and middle-income countries (LMICs) gave an IRR of 2.09 (CI, 1.39–3.14), compared with 1.66 (95% CI, 1.13–2.45) in non-LMICs. In HBCs, IRR was 2.44 (95% CI, 1.67–3.54), compared with 1.50 (95% CI, 1.10–2.04) in non-HBCs. Finally, in countries with a high burden of TB/HIV coinfection, IRR was 2.44 (95% CI, 1.69–3.54); in those without a high burden of TB/HIV coinfection, IRR was 1.50 (95% CI, 1.10–2.04).
Figure 3.
Forest plot showing pooled incidence rate ratio (IRR) for active tuberculosis among healthcare workers (HCWs). Abbreviation: CI, confidence interval.
DISCUSSION
This study provides an update to previous work [6–8] and is the first meta-analysis to restrict analyses to studies that included control groups. This allows direct comparison of HCWs with the local population, providing a more reliable estimate of their relative risk for LTBI and active TB. The pooled prevalence estimate for LTBI in HCWs was 37% in our study, and the risk of LTBI among HCWs is more than twice that of control populations. The prevalence findings are lower than previously published reports [7, 8], and our risk to HCWs of LTBI compared with the general population is of a similar magnitude to previous findings [7, 37]. For example, a review published in 2007 found that HCWs had a 2–3 times increased morbidity risk when matched for employment and socioeconomic status [8], and the studies in our analysis that compared HCWs and school workers found the risk to HCWs to be approximately double. Our findings are consistent with previous studies, but we found a reduced overall prevalence of LTBI in HCWs, which is consistent with a decrease among the general population, as found in the most recent WHO TB report [1]. We unexpectedly found that the risk decreased when the analysis was restricted to LMICs, possibly due to the small number of studies included in our meta-analysis, which may not give a comprehensive view of risk in LMICs. A 2008 review found a lower risk in HICs; the annual incidence of TB infection attributable to working in healthcare was 1.1% in HICs compared with 5.8% in LMICs [8]. The considerable heterogeneity in our analysis, although a limitation, may also may highlight the large variation in LTBI risk to HCWs.
HCWs were found to have an approximately 3 times greater risk of active TB compared with the general population, although there was substantial variability between studies. The findings illustrate how TB estimates may vary considerably within and between countries: for example, 2 studies in China and Taiwan showed IRs of active TB to be 78.3% and 61.1%, respectively [17, 30]. In addition, while looking at the prevalence of LTBI, 2 studies in Brazil showed very different prevalence estimates of 18.4% and 59.8% [21, 22]. These findings suggest that adaptation of national infection control policies may be required at the regional level.
Among the studies investigating prevalence of LTBI, a significant reduction in heterogeneity was only observed when the analysis was restricted to studies using IGRA. This increased the estimated risk to HCWs, as a result of a reduction in cases among the control group. Although this finding suggests that IGRA is a more specific and discriminatory tool among the general population, there appear to be limitations for its use in screening HCWs. A recent study found that the rate of positive results among HCWs in a low-burden setting was greatly increased when screening was switched from TST to IGRA, and subsequent follow-up using IGRA produced a significant reversion rate in those who had originally tested positive [38]. This demonstrates the low reproducibility of IGRA among HCWs, which has been corroborated in other settings with a low TB burden [39]. Therefore, our finding that IGRA appeared to be more discriminatory in areas of a high TB burden cannot be applied to all settings, highlighting that further research is needed to determine TB screening in specific populations.
There are a number of limitations to note. First, there was substantial heterogeneity, reflecting the different settings and populations included in the review. Second, there appears to have been an element of publication bias with a paucity of small studies showing negative results, possibly leading to an overestimation of the TB risk to HCWs. The low number of studies and significant heterogeneity between them reflects the limited evidence base and highlights the need for high-quality, comparative studies. Few studies recorded information on important confounders such as Bacillus Calmette-Guérin vaccination status, magnitude of TB exposure, and extent of infection control policies. Finally, including only English-language studies may have resulted in a language bias in our search; nevertheless, studies included here cover all continents where TB is a significant, occupational concern.
CONCLUSIONS
In conclusion, the most recent data show that HCWs globally remain at increased risk of both latent and active TB compared with the general population, despite an absolute decrease in TB prevalence. These findings should encourage even greater attention to prevention measures and screening for HCWs in all settings as part of efforts to achieve the WHO targets for 2030 [23].
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
Financial support. G. S. C. received partial funding from the National Institute for Health Research Biomedical Research Centre of Imperial College NHS Trust. E. B. received funding from the Médecins Sans Frontières.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis report 2016 Available at: http://www.who.int/tb/publications/global_report/en/. Accessed 8 Febuary 2017.
- 2. World Health Organization. The END TB strategy Available at: http://www.who.int/tb/End_TB_brochure.pdf. Accessed 13 April 2016.
- 3. Corbett EL, Muzangwa J, Chaka K et al. . Nursing and community rates of Mycobacterium tuberculosis infection among students in Harare, Zimbabwe. Clin Infect Dis 2007; 44:317–23. [DOI] [PubMed] [Google Scholar]
- 4. Williams OM, Abeel T, Casali N et al. . Fatal nosocomial MDR TB identified through routine genetic analysis and whole-genome sequencing. Emerg Infect Dis 2015; 21:1082–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walker TM, Crook DW, Peto TE, Conlon CP. Whole-genome sequencing identifies nosocomial transmission of extra-pulmonary M. tuberculosis. QJM 2016; 109:819–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baussano I, Nunn P, Williams B et al. . Tuberculosis among health care workers. Emerg Infect Dis 2011; 17:488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joshi R, Reingold AL, Menzies D, Pai M. Tuberculosis among health-care workers in low- and middle-income countries: a systematic review. PLoS Med 2006; 3:e494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Menzies D, Joshi R, Pai M. Risk of tuberculosis infection and disease associated with work in health care settings. Int J Tuberc Lung Dis 2007; 11:593–605. [PubMed] [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. Counting health workers: definitions, data, methods and global results Available at: http://www.who.int/hrh/documents/counting_health_workers.pdf. Accessed 14 April 2016.
- 11. Corbett EL, Watt CJ, Walker N et al. . The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med 2003; 163:1009–21. [DOI] [PubMed] [Google Scholar]
- 12. Venkata RK, Kumar S, Krishna RP et al. . Tuberculosis in chronic kidney disease. Clin Nephrol 2007; 67:217–20. [DOI] [PubMed] [Google Scholar]
- 13. Stevenson CR, Forouhi NG, Roglic G et al. . Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health 2007; 7:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vandenbroucke JP, von Elm E, Altman DG et al. . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 2007; 4:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization. Use of high TB burden country lists in the post-2015 era (Discussion Paper) Available at: http://www.who.int/tb/publications/global_report/high_tb_burdencountrylists2016-2020.pdf. Accessed 29 April 2016.
- 16. The World Bank. Income Group Data. 2015 Available at: http://data.worldbank.org/country. Accessed 29 April 2016.
- 17. Chu H, Shih CJ, Lee YJ et al. . Risk of tuberculosis among healthcare workers in an intermediate-burden country: a nationwide population study. J Infect 2014; 69:525–32. [DOI] [PubMed] [Google Scholar]
- 18. Agaya J, Nnadi CD, Odhiambo J et al. . Tuberculosis and latent tuberculosis infection among healthcare workers in Kisumu, Kenya. Trop Med Int Health 2015; 20:1797–804. [DOI] [PubMed] [Google Scholar]
- 19. Drobniewski F, Balabanova Y, Zakamova E et al. . Rates of latent tuberculosis in health care staff in Russia. PLoS Med 2007; 4:e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Durando P, Sotgiu G, Spigno F et al. . Latent tuberculosis infection and associated risk factors among undergraduate healthcare students in Italy: a cross-sectional study. BMC Infect Dis 2013; 13:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Franco C, Zanetta DM. Assessing occupational exposure as risk for tuberculous infection at a teaching hospital in Sao Paulo, Brazil. Int J Tuberc Lung Dis 2006; 10:384–9. [PubMed] [Google Scholar]
- 22. Maciel EL, Meireles W, Silva AP et al. . Nosocomial Mycobacterium tuberculosis transmission among healthcare students in a high incidence region, in Vitoria, State of Espirito Santo. Rev Soc Bras Med Trop 2007; 40:397–9. [DOI] [PubMed] [Google Scholar]
- 23. Sawhney N, Mehta S, Singh VA, Shinu P. Application of tuberculin skin test in diagnosis of latent tuberculosis: a two year experience in a tertiary care hospital. J Pharm Biomed Sci 2015; 05:643–9. [Google Scholar]
- 24. Nikokar I, Dadgran A, Mafozei L. A Comparison of two-step tuberculin skin test between health-care workers and nonhospital employees. Iran J Med Sci 2015; 35:201–4. [Google Scholar]
- 25. Powell K, Han D, Hung NV et al. . Prevalence and risk factors for tuberculosis infection among personnel in two hospitals in Viet Nam. Int J Tuberc Lung Dis 2011; 15:1643–9. [DOI] [PubMed] [Google Scholar]
- 26. Rutanga C, Lowrance DW, Oeltmann JE et al. . Latent tuberculosis infection and associated factors among health care workers in Kigali, Rwanda. PLoS One 2015; 10:e0124485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Storla DG, Kristiansen I, Oftung F et al. . Use of interferon gamma-based assay to diagnose tuberculosis infection in health care workers after short term exposure. BMC Infect Dis 2009; 9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu C, Liu Z, Li Z, Mei S, Hu Z. The performance and limitation of T-SPOT. TB for the diagnosis of TB in a high prevalence setting. J Thorac Dis 2014; 6:713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Rie A, McCarthy K, Scott L et al. . Prevalence, risk factors and risk perception of tuberculosis infection among medical students and healthcare workers in Johannesburg, South Africa. S Afr Med J 2013; 103:853–7. [DOI] [PubMed] [Google Scholar]
- 30. Chen B, Wang X, Zhong J et al. . Tuberculosis among healthcare workers in southeastern China: a retrospective study of 7-year surveillance data. Int J Environ Res Public Health 2014; 11:12042–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Claassens MM, Sismanidis C, Lawrence KA et al. . Tuberculosis among community-based health care researchers. Int J Tuberc Lung Dis 2010; 14:1576–81. [PubMed] [Google Scholar]
- 32. Klimuk D, Hurevich H, Harries AD et al. . Tuberculosis in health care workers in Belarus. Public Health Action 2014; 4(Suppl 2):S29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pan SC, Chen YC, Wang JY et al. . Tuberculosis in healthcare workers: a matched cohort study in Taiwan. PLoS One 2015; 10:e0145047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pazin-Filho A, Soares CS, Ferrais AdSN et al. . Tuberculosis among health care workers in a Brazilian tertiary hospital emergency unit. Am J Emerg Med 2008; 26:796–8. [DOI] [PubMed] [Google Scholar]
- 35. Skodric-Trifunovic V, Markovic-Denic L, Nagorni-Obradovic L, Vlajinac H, Woeltje KF. The risk of occupational tuberculosis in Serbian health care workers. Int J Tuberc Lung Dis 2009; 13:640–4. [PubMed] [Google Scholar]
- 36. Sotgiu G, Arbore AS, Cojocariu V et al. . High risk of tuberculosis in health care workers in Romania. Int J Tuberc Lung Dis 2008; 12:606–11. [PubMed] [Google Scholar]
- 37. Zwerling A, van den Hof S, Scholten J et al. . Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax 2012; 67:62–70. [DOI] [PubMed] [Google Scholar]
- 38. Joshi M, Monson TP, Joshi A, Woods GL. IFN-γ release assay conversions and reversions. Challenges with serial testing in U.S. health care workers. Ann Am Thorac Soc 2014; 11:296–302. [DOI] [PubMed] [Google Scholar]
- 39. Pai M, Banaei N. Occupational screening of health care workers for tuberculosis infection: tuberculin skin testing or interferon-γ release assays? Occup Med 2013; 63:458–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.