Abstract
The homeostatic regulation of feeding behavior requires an organism to be able to integrate information from its internal environment, including peripheral visceral signals about the body's current energy needs, with information from its external environment, such as the palatability of energy‐rich food stimuli. The insula, which serves as the brain's primary sensory cortex for representing both visceral signals from the body and taste signals from the mouth and tongue, is a likely candidate region in which this integration might occur. However, to date it has been unclear whether information from these two homeostatically critical faculties is merely co‐represented in the human insula, or actually integrated there. Recent functional neuroimaging evidence of a common substrate for visceral interoception and taste perception within the human dorsal mid‐insula suggests a model whereby a single population of neurons may integrate viscerosensory and gustatory signals. To test this model, we used fMRI‐Adaptation to identify whether insula regions that exhibit repetition suppression following repeated interoception trials would then also exhibit adapted responses to subsequent gustatory stimuli. Multiple mid and anterior regions of the insula exhibited adaptation to interoceptive trials specifically, but only the dorsal mid‐insula regions exhibited an adapted gustatory response following interoception. The discovery of this gustatory‐interoceptive convergence within the neurons of the human insula supports the existence of a heretofore‐undocumented neural pathway by which visceral signals from the periphery modulate the activity of brain regions involved in feeding behavior. Hum Brain Mapp 38:2150–2164, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: interoception, gustation, insular cortex, fMRI, fMRI‐adaptation
INTRODUCTION
The insula serves as the primary cortical destination for afferent gustatory signals as well as afferent interoceptive signals from throughout the entire gastro‐intestinal tract, via projections from relay nuclei in the brainstem and thalamus [Berthoud and Neuhuber, 2000; Craig, 2002; Pritchard et al., 1986]. As such, it is a prime candidate region for integrating internal and external sensory stimuli related to maintaining energy homeostasis [de Araujo et al., 2012]. Although electrophysiological studies in rodents have found evidence of both distinct and shared gustatory/interoceptive representation in the insula [Hanamori et al., 1998b; Ogawa and Wang, 2002], electrophysiology studies of non‐human primates have only observed distinct gustatory and interoceptive processing centers [Scott and Plata‐Salaman, 1999; Zhang et al., 1998]. Electrophysiological studies, however, are limited in their ability to survey simultaneously the response of large expanses of cortex, so it remains unclear whether primates have retained the neural hardware for direct gustatory‐interoceptive integration. Recent human neuroimaging studies, however, have provided evidence that activity in subregions of the mid‐insula is modulated by both gustatory and interoceptive tasks [Avery et al., 2015] (Fig. 1).
Figure 1.
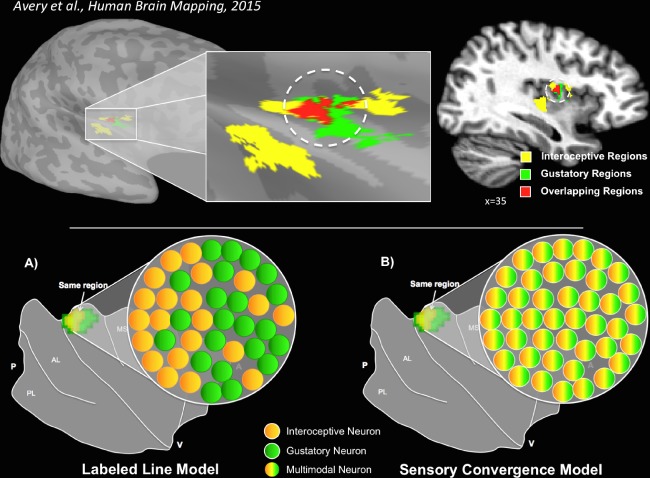
Interoceptive and gustatory co‐activation of the dorsal mid‐insula. Top: A single group of subjects, performing very different tasks, displayed overlapping activation patterns in an identical region of the dorsal mid‐insula [Avery et al., 2015]. The image on the left depicts overlapping activations (red) on the cortical surface of the right dorsal mid‐insula when subjects performed a task requiring interoceptive attention to visceral sensations (yellow) or received sweet and neutral tastants during scanning (green). The image on the right depicts the same results, transformed into volumetric space for display purposes. Bottom: Two potential models for this gustatory‐interoceptive overlap. (A) Labeled Line Model—the voxels within that region of the insula contain groups of neurons separately responsible for either gustatory or interoceptive processing. (B) Sensory Convergence Model—the voxels within that region of the insula contain groups of multimodal neurons that support a shared representation of both gustation and interoception. [Color figure can be viewed at http://wileyonlinelibrary.com]
There are two potential models for explaining this apparent gustatory‐interoceptive overlap (Fig. 1, bottom). A labeled‐line model would predict that, within regions of the insula co‐activated by gustation and interoception, there exist co‐mingled but distinct pools of modality‐specific neurons responsible for either gustatory or interoceptive processing. Alternatively, a sensory convergence model would predict that these regions of the insula contain groups of multimodal neurons co‐activated by both gustation and interoception. This latter possibility is supported by electrophysiological studies identifying populations of neurons within the rat insular cortex multi‐modally responsive to visceral, nociceptive, and gustatory stimulation [Hanamori et al., 1998b]. Importantly, these two competing accounts cannot be distinguished due to the spatial resolution limits of fMRI, but their distinct response properties should be detectable using an fMRI‐Adaptation paradigm (fMRI‐A) [Grill‐Spector and Malach, 2001]. Convergence of gustatory and interoceptive information within the neurons of the human mid‐insula may reflect a novel pathway by which visceral signals from the periphery modulate the activity of brain regions involved in feeding behavior, potentially accounting for the homeostatic responses to food pictures observed within caudal insular cortex [Simmons et al., 2013b].
To evaluate between these two models of gustatory‐interoceptive co‐representation within the insula, we recruited healthy participants to perform the Gustatory‐Interoceptive Adaptation (GIA) task, an fMRI‐A paradigm (Fig. 2) designed to identify neuronal populations supporting the shared representation of gustatory and interoceptive processing. fMRI‐A paradigms take advantage of the phenomenon in which repeated presentations of identical stimuli cause short‐term decreases in the activity of neurons sensitive to those stimuli (for reviews, see [Gotts et al., 2012; Grill‐Spector et al., 2006]). Selectivity of neurons within an fMRI voxel can then be probed indirectly by presenting a stimulus that shares certain properties and a proportion of cells/synapses with the adapted stimulus, and then observing the relative recovery from adaptation. The GIA task combined visual stimulus presentation and gustatory stimulus presentation using an MR‐compatible tastant delivery system. During the GIA task, successive repetitions (i.e., adaptation trains) of an interoceptive stimulus (interoceptive attention to heartbeat sensations), as well as an exteroceptive control stimulus (exteroceptive attention to a visual stimulus), were employed to adapt the brain's response to either condition. Adaptation trains were then followed by tastant delivery, allowing us to examine differential neural responses to gustatory stimulation following interoceptive or exteroceptive adaptation. According to the Sensory Convergence model (Fig. 1), we would expect to observe one or more regions within the insula that (A) are specifically responsive to visceral interoception as well as gustatory stimulation and (B) exhibit suppressed responses to gustatory stimuli that follow interoceptive adaptation trains, relative to gustatory stimuli that follow exteroceptive adaptation trains.
Figure 2.
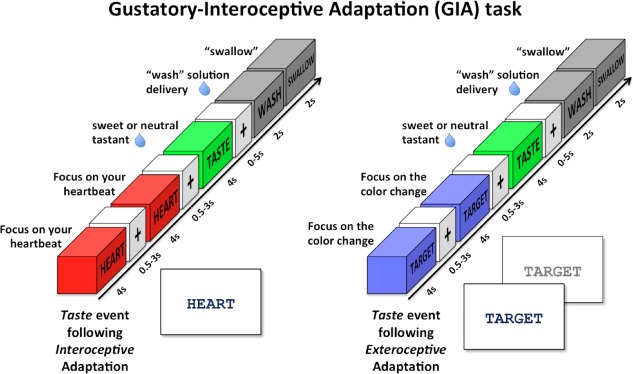
Gustatory‐interoceptive adaptation (GIA) task. The GIA task was composed of Interoception and Exteroception events presented in rapid succession (adaptation trains) in order to adapt the response of Taste events following those trains. During Interoception events, subjects focused on how intensely they could feel the sensation of their heart beating while the word “HEART” was presented on the screen. During Exteroception events, the word “TARGET” was presented in the middle of the screen and the font color would change to a lighter shade of gray every 500 ms. Subjects were instructed to focus their attention on the intensity of these color changes. Taste events involved the delivery of a sweet (0.4 mL of 0.6 M sucrose) or neutral (0.4 mL of distilled water) tasting liquid followed by a Wash period to wash the liquid from the subject's mouth. All Interoception, Exteroception, and Taste events were separated by variable duration interstimulus intervals (i.e. ‘jitters’) of 0.5 s to 3.0 s. These adaptation‐stimulus blocks were arranged in random order throughout each run of the GIA task. [Color figure can be viewed at http://wileyonlinelibrary.com]
METHODS AND MATERIALS
Subjects
Fifteen right‐handed, native English‐speaking volunteers (eight female; age: mean(SD) = 29(10), range = 18–44; body mass index (BMI): mean(SD) = 25(5), range = 19–34). All subjects underwent clinical assessment prior to participating in the study including a Structured Clinical Interview for DSM‐IV Axis‐I Disorders (SCID‐I) conducted by trained Master's level clinicians with experience in psychiatric diagnosis. Exclusion criteria for this study included: any major psychiatric disorder on the SCID‐I, any major medical or neurological disorder, a past history of traumatic brain injury, current pregnancy, any history of substance abuse, use of psychotropic medications, or recent exposure to other drugs likely to affect cerebral function or blood flow within 3 weeks (6 weeks for fluoxetine). All subjects were paid for their participation and provided written informed consent as approved by an Institutional Review Board.
Experimental Design
Each subject received a structural MRI scan followed by a series of functional MRI scans, during which they performed the Gustatory Mapping (GM) and Gustation‐Interoception Adaptation (GIA) task.
Gustation‐interoception adaptation task
This task consisted of four basic events: Interoception events, exteroception events, taste events, and wash events (Fig. 2). Each of these events occurred within 4 s throughout each of four, 630 s GIA task scans. Visual stimuli were projected onto a screen located inside the scanner bore and viewed through a mirror system mounted on the head‐coil. The visual cues for each GIA task event were presented in black font against a white background. Stimulus presentation was controlled using E‐Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA).
During interoception events, subjects saw the word “HEART” in the center of the screen and they focused on how intensely they could feel the sensation of their heart beating (Fig. 2). This condition is designed to take advantage of the attentional spotlight effect by requiring participants to focus their attention on their naturally occurring interoceptive sensations. This effect has been previously demonstrated in other sensory modalities such as touch and taste [Johansen‐Berg et al., 2000; Veldhuizen et al., 2007] to amplify the signal within cortical regions underlying those sensory modalities. The effectiveness of this task at mapping interoceptive regions of the insula has been likewise demonstrated in previous studies, across multiple interoceptive modalities [Avery et al., 2014, 2015; Kerr et al., 2016; Simmons et al., 2013a]. Importantly, this study exclusively used heartbeat interoception, rather than stomach interoception, in order to rule out any potential adaptation effects occurring due to the semantic similarity between the related concepts of “TASTE” and “STOMACH”.
During exteroception events, the word “TARGET” was presented in the middle of the screen and the font color of the word alternated between black and a lighter shade of gray every 500 ms (Fig. 2). The intensity of this color change (from a 15% to 87% gray‐scale shift) was fixed for each individual 4 s event, but would vary between events. The subjects were instructed to focus their attention on the intensity of these color changes while the word was presented on the screen. This condition was specifically designed as a control for the interoceptive “HEART” condition, as it requires participants to attend to an externally presented stimulus with periodically fluctuating intensity during each trial.
During taste events, the subjects saw the word “TASTE” appear on the screen for 4 s, at which time they received either a sweet (0.4 mL of 0.6 M glucose) or neutral tastant (0.4 mL of distilled water) (Fig. 2). Subjects were instructed to let the solution roll down on their tongue, but not to swallow. During wash events, the word “WASH” appeared for 2 s and 0.8 mL of distilled water was delivered onto the subject's tongue. Immediately after this, the word “SWALLOW” appeared for 2 s, directing the subject to swallow. All taste events were followed by wash events, after a variable duration inter‐stimulus interval (ISI) (between 0 and 5 s), during which time subjects saw only a black fixation mark against a white background. Though Wash events occurred after all Taste events, they also occurred independently of Taste events, in order to reduce the serial correlation of these distinct events and allow for separately modeling their responses. Postscan ratings of the tastants delivered during the GIA task indicated that subjects perceived the taste of the sucrose as significantly sweeter (t (14) = 11.46, P < 0.001) and more intense (t (14) = 6.28; P < 0.001), but not more pleasant (t (14) = 1.03; P = 0.32) than the neutral tastant.
Within the GIA task, interoception and exteroception events were presented in “adaptation trains,” composed of two events of the same type paired in rapid succession (Fig. 2). These adaptation trains were designed to decrease or adapt the BOLD response of the following taste trial [Grill‐Spector and Malach, 2001]. The two interoception or exteroception events composing the adaptation train were separated by a variable duration ISI of 0.5 s to 3.0 s (mean 1.75 s), which facilitated the separate modeling of those individual events (aka “jittering” [Dale, 1999]). The GIA task incorporated 12 Interoception and 12 Exteroception adaptation trains during each of the four runs of this task (48 total for both train types). Interoception events within the GIA task occurred both within adaptation trains and directly after both Interoception and Exteroception adaptation trains. The freestanding interoception events (eight events/run; 32 total) were included in order to (A) account for potential expectation effects, such that adaptation trains would not always be followed by taste events; and (B) to reduce the possibility of serial autocorrelation between adaptation train events and taste events (i.e. to ensure the responses to these events were modeled separately within the fMRI time‐series). There were no freestanding exteroception events within this task.
All Taste events in the GIA task occurred after interoception or exteroception adaptation trains, separated by a variable duration ISI of 0.5 s to 3.0 s (mean 1.75 s). Half of the Taste events occurred after Interoception adaptation trains (eight events/run) and half occurred after Exteroception adaptation trains (eight events/run). Additionally, the Taste events occurring after these adaptation trains were evenly divided into sweet and neutral Taste events (16 Taste events/run: eight sweet + eight neutral; 64 total Taste events). These adaptation‐stimulus blocks were arranged in random order throughout each run of the GIA task.
All solutions used for the GIA task were delivered via a MRI‐compatible tastant delivery system. Solutions were kept at room temperature in four separate syringe pumps (one for sweet, one for neutral, two for wash) and delivered to the participant via plastic tubing connected to a gustatory manifold. This manifold was anchored to the head coil in the scanner and delivered solutions directly into the participant's mouth during scanning. A laptop using LabView (National Instruments, Austin, TX) software enabled precise timing and delivery of all solutions.
Gustatory mapping task
This task involved both Taste events as well as Wash events as described above. The GM task additionally contained Cue events. During Cue events, the word “SWEET” or “NEUTRAL” appeared on the screen for 5 s. In the GM task, each Taste trial was preceded by a Cue trial, but 40% of the Cue trials were not followed by Taste trials. These freestanding Cue trials enabled the response to the word cues to be separately modeled from the response to the Tastant trials. During the GM task, Taste, Cue, and Wash events were presented in 5 s blocks. Please see Avery et al. (2015) for a more detailed description of the GM task design. Following the completion of fMRI scanning, participants were asked to make ratings of the perceived sweetness, intensity, and pleasantness of the sweet and neutral tastants delivered during the Gustatory Mapping and GIA tasks. These items were rated on a scale ranging from 1 (not sweet/intense/pleasant) to 10 (extremely sweet/intense/pleasant).
MRI Imaging Parameters
Magnetic resonance images were collected in a General Electric Discovery MR750 3 T MRI scanner (GE Healthcare, Milwaukee, WI) using a scalable 32‐channel digital MRI receiver capable of performing massively‐parallel fMRI. A brain‐dedicated receive‐only 32‐element coil array (Nova Medical Inc., Wilmington, MA) optimized for parallel imaging, was used for MRI signal reception. A single‐shot gradient‐recalled echo‐planar imaging (EPI) sequence with Sensitivity Encoding (SENSE) depicting blood oxygenation level dependent (BOLD) contrast was used for functional scans. A T1‐weighted magnetization‐prepared rapid acquisition gradient‐echo (MPRAGE) sequence with SENSE was used to provide an anatomical reference for the fMRI analysis.
EPI imaging parameters (GM and GIA task)
FOV/slice/gap = 240/2.9/0 mm, 46 axial slices/volume, acquisition matrix = 96 × 96 (for an effective EPI resolution of 2.5 × 2.5 × 2.9 mm3), repetition/echo time TR/TE = 2,500/30 ms, SENSE acceleration factor R = 2 in the phase encoding (anterior‐posterior) direction, flip angle = 90°, sampling bandwidth = 250 kHz. The EPI images were reconstructed into a 128 × 128 matrix, resulting in an fMRI voxel volume of 1.875 × 1.875 × 2.9 mm³. The GM Task was collected during four EPI scans (number of volumes = 248/scan, total scan time 620 s/scan). The GIA Task was also collected during four scanning runs (number of volumes = 252/scan, scan time 630 s/scan).
Anatomical imaging parameters
FOV = 240 mm, axial slices/volume = 176, slice thickness = 0.9 mm, image matrix = 256 × 256, voxel volume = 0.938 × 0.938 × 0.9 mm³, TR/TE = 5/2.02 ms, acceleration factor R = 2, flip angle = 8°, inversion/delay time TI/TD = 725/14,00 ms, sampling bandwidth = 31.25 kHz, scan time = 372 s.
fMRI image preprocessing
Identical image preprocessing and motion correction procedures were performed for data from both the Gustatory Mapping and GIA tasks. We analyzed the unsmoothed fMRI data from both tasks on a standardized cortical surface model, in order to improve inter‐subject alignment and reduce the variability in functional topography due to individual differences in cortical folding patterns [Van Essen and Drury, 1997]. Prior to statistical analyses, image preprocessing was performed using AFNI and SUMA (http://afni.nimh.nih.gov/afni), as well as FreeSurfer (http://surfer.nmr.mgh.harvard.edu/) for anatomical surface construction and parcellation. The anatomical scan was registered to the first volume of the first EPI time‐course and was then transformed into an anatomical surface model that contained an identical number of cortical surface nodes (156,252 nodes/hemisphere) and identical node indices across subjects [Argall et al., 2006]. The first four volumes of each EPI time‐course (10 s) were excluded from data analysis to allow the fMRI signal to reach longitudinal equilibrium and a slice timing correction was applied to the remaining 248 volumes of each EPI scan. All EPI volumes were then registered to the first volume of the EPI time‐course using a six‐parameter (three translations, three rotations) motion correction algorithm, and the motion estimates were saved for use as regressors in the subsequent statistical analyses. Immediately following volume registration, the EPI data were then transformed and mapped to the standardized cortical surface using the SUMA program, 3dVol2Surf. This transformation of the functional images to a standardized cortical surface template allowed for significant improvement of cortical surface alignment across subjects and reduction in the variability in functional topography due to individual differences in cortical folding patterns [Argall et al., 2006]. Additionally, no spatial smoothing was applied to the EPI data, which might otherwise artificially magnify or diminish adaptation responses present within neighboring voxels of the insula through spatial averaging of the MR signal. After transformation of the EPI data to the cortical surface, the signal intensity for each EPI volume was normalized to reflect percent signal change from each voxel's mean intensity across the time‐course.
Additional motion correction
In order to remove any additional motion related signal artifacts that were still present after regression of motion parameters, a censoring technique was implemented to identify and remove any time point with motion above a certain predefined threshold. The AFNI program 1d_tool.py was used on the six motion parameters created during the volume registration step. The output was a single time series reflecting the Euclidean normalized derivative of the motion parameters. This time series was then thresholded, so that any time point where the derivative (i.e. the change across consecutive time points) was greater than 0.3 (roughly 0.3 mm motion) was censored. Time points censored per subject − [mean%(SD): 7%(5); range: 0–17%]. The list of censored time points was then provided to the AFNI program 3dDeconvolve, which removed those time points from consideration during the subsequent regression analysis.
Statistical Analyses
At the subject level, a multiple linear regression model was constructed, via the AFNI program 3dDeconvolve, to examine the task data. For the Gustatory Mapping task the regression model included regressors for the sweet cue, neutral cue, sweet tastant, neutral tastant, and wash/swallow events. The five task regressors were constructed by convolution of a canonical hemodynamic response function with a gamma‐variate function represented at the onset of each occurrence of each type of trial. Additionally, the regression model for both tasks included regressors of non‐interest to account for each run's signal mean, linear, quadratic, and cubic signal trends, as well as the six normalized motion parameters (three translations, three rotations) computed during the image registration preprocessing.
For the GIA task, the model included regressors for each task condition, regressors of noninterest to account for each run's signal mean, linear, quadratic, and cubic signal trends, and the six normalized motion parameters (three translations, three rotations) computed during the image registration preprocessing. The regressors for each task condition (Interoception, Exteroception, Taste, and Wash) were constructed by convolution of a canonical hemodynamic response function with a gamma‐variate function represented at the onset of each occurrence of each GIA task event. The individual events comprising the Interoception (I) and Exteroception (E) adaptation trains were modeled separately (denoted by the subscript A or B, depending upon their order in the adaptation train). Sweet and neutral Taste events were also modeled separately as SWEET events and NEUTRAL events. In order to examine the effect of adaptation type on SWEET and NEUTRAL events, these events were further divided into groups and modeled separately in the design matrix, according to whether they occurred after an Interoception or Exteroception adaptation train (denoted by the subscript EA for Exteroception adaptation and IA for Interoception adaptation).
Model Evaluation
Evaluating between the two competing models of gustatory‐interoceptive co‐activation (see Fig. 1) requires first the identification of regions of the brain activated by both sensory modalities. Using the GIA task data, a two‐step procedure was performed to identify cortical regions that were selectively adapted by Interoception events. First, the individual regression coefficients produced by 3dDeconvolve at the subject level were combined in a group level random‐effects analysis on the cortical surface using the AFNI program 3dttest++ to examine group‐level adaptation effects. The Interoceptive Adaptation contrast, I A − I B (which represents the hemodynamic response of the first event of the Interoception adaptation train minus the second) was used to identify regions of the cortical surface exhibiting significant adaptation to consecutively presented Interoception events. The Exteroceptive Adaptation contrast E A − E B (again, the hemodynamic response of the first event of the Exteroception adaptation train minus the second) was used to identify cortical regions displaying significant adaptation to consecutively presented Exteroception events. Both contrast maps were then whole‐brain FDR corrected for multiple comparisons (FDR q < 0.05). Subsequently, both adaptation contrast maps were transformed into binary valued masks and a conjunction of the two masks was created. Voxels exhibiting selective adaptation following interoception events were first identified by removing any voxels from the mask that also exhibited significant adaptation to exteroception (Table 1). Second, the remaining voxels were clustered into regions and further subjected to 2 (Interoception vs. Exteroception) × 2 (first vs. second) interaction tests to confirm that significantly greater adaptation was observed on Interoception trials (Fig. 3, Table 2).
Table 1.
Cortical regions exhibiting adaptation to interoception and not exteroception events—Step 1
| Peak coordinatesb | |||||
|---|---|---|---|---|---|
| Side/locationa | X | Y | Z | t 14 | Volume (mm3) |
| L dorsal mid‐insula | −30 | −8 | +14 | 3.78 | 183 |
| L ventral insula | −40 | +4 | −8 | 4.07 | 106 |
| L dorsal anterior insula | −30 | +6 | +12 | 3.22 | 86 |
| R dorsal mid‐insula | +33 | −7 | +16 | 3.84 | 68 |
| R dorsal anterior insula | +32 | +6 | +15 | 3.78 | 40 |
| R anterior cingulate cortex | +3 | +37 | +18 | 4.53 | 565 |
| L anterior cingulate cortex | −2 | +29 | +27 | 3.14 | 145 |
| L medial frontal gyrus | −11 | +31 | +33 | 4.26 | 48 |
All reported brain regions were first identified using a standardized cortical surface model, then subsequently transformed into volumetric space.
All coordinates reported according to Talairach stereotaxic atlas.
Figure 3.
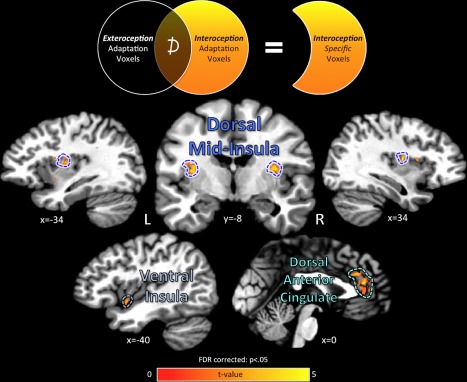
Cortical regions exhibiting interoception‐specific adaptation. Regions of the cerebral cortex exhibiting a specific adaptation response to successive repetitions of Interoception events were located after subtracting out any cortical regions also displaying adaptation to Exteroception control events. Interoception‐specific regions were located in the bilateral dorsal mid‐insula as well as the left ventral insula. Outside of the insula, the bilateral dorsal anterior cingulate cortex also displayed specific adaptation for interoceptive attention. The unsmoothed fMRI data were plotted and analyzed on a standardized cortical surface model, and statistical contrasts were FDR corrected for multiple comparisons at P < 0.05. Data is plotted in volumetric space for viewing purposes. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Cortical regions exhibiting adaptation to interoception and not exteroception events—Step 2
| Interoceptive adaptation (I A − I B) | Exteroceptive adaptation (E A − E B) | Interoception‐Exteroceptiona | ||||
|---|---|---|---|---|---|---|
| % Signal change | % Signal change | % Signal change | ||||
| Mean | SD | Mean | SD | t (14) | P | |
| Adaptation magnitude | ||||||
| L dorsal mid‐insula | 0.17 | 0.18 | 0.07 | 0.16 | 2.21 | 0.04 |
| L ventral insula | 0.20 | 0.25 | 0.07 | 0.20 | 2.56 | 0.02 |
| L dorsal anterior insula | 0.15 | 0.20 | 0.08 | 0.17 | 1.47 | 0.16 |
| R dorsal mid‐insula | 0.24 | 0.26 | 0.11 | 0.26 | 2.32 | 0.04 |
| R dorsal anterior insula | 0.21 | 0.24 | 0.12 | 0.22 | 1.37 | 0.19 |
| R anterior cingulate cortex | 0.16 | 0.15 | 0.05 | 0.14 | 3.19 | 0.01 |
| L anterior cingulate cortex | 0.17 | 0.21 | 0.05 | 0.17 | 2.15 | 0.05 |
| L medial frontal gyrus | 0.14 | 0.16 | 0.07 | 0.17 | 1.24 | 0.24 |
t values were computed using two‐tailed paired t‐tests.
The next step was to identify whether those interoceptive‐specific regions of the cortical surface were also responsive to gustatory stimulation. This was done by examining data from the Gustatory Mapping (GM) task performed by each subject immediately prior to the GIA task. Within each interoception‐specific ROI on the cortical surface, the average value of each subject's beta coefficients for the sweet and neutral stimulus conditions from the GM task was extracted and a group‐level pairwise t‐test of those coefficients was performed (Fig. 4, Table 3).
Figure 4.
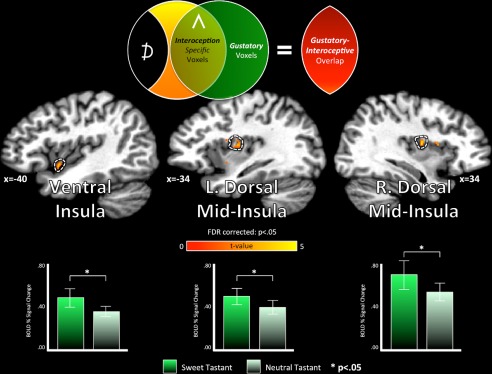
Gustatory and interoceptive co‐activation. Prior to the GIA task, subjects performed a Gustatory Mapping (GM) task, which involved the presentation of sweet and neutral tastants during scanning [Avery et al. 2015; see Methods section for details]. Using imaging data acquired during the GM task, we next examined whether those interoceptive‐specific regions of the brain identified using the GIA task (see Fig. 3, Table 3) were also co‐activated by gustatory stimulation. Within each of those cortical surface regions, we compared the hemodynamic response to sweet vs. neutral tastants (t (14), two‐tailed paired t‐test). Neither region of the anterior cingulate cortex exhibited a significant response to the tastants, nor did their activity discriminate between them (P > 0.24; see Table 3). Only the ventral and mid‐insula regions exhibited co‐activation for gustatory and interoceptive processing. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Gustatory responses during the GM task within interoception‐specific regions of the brain
| Sweet tastant | Neutral tastant | Sweet‐neutrala | ||||||
|---|---|---|---|---|---|---|---|---|
| brain region | % Signal change | % Signal change | % Signal change | |||||
| Interoception > exteroception | Mean(SD) | t | P | Mean(SD) | t | P | t (14) | P |
| L dorsal mid‐insula | 0.49(0.29) | 6.60 | <0.001 | 0.39(0.24) | 6.24 | <0.001 | 2.63 | <0.02 |
| L ventral insula | 0.48(0.32) | 5.79 | <0.001 | 0.35(0.18) | 7.65 | <0.001 | 2.11 | <0.05 |
| R dorsal mid‐insula | 0.69(0.51) | 5.24 | <0.001 | 0.54(0.33) | 6.27 | <0.001 | 2.18 | <0.05 |
| R Anterior Cingulate | −0.07(0.09) | −3.03 | 0.01 | −0.05(0.10) | −1.97 | 0.07 | −1.22 | 0.24 |
| L Anterior Cingulate | 0.00(0.11) | −0.13 | 0.89 | −0.04(0.09) | −1.55 | 0.14 | 1.06 | 0.31 |
t values were computed using paired t‐tests.
Finally, a further series of analyses was performed using the GIA task data, to evaluate between the two competing models. The goal of this final set of analyses was to identify whether interoceptive adaptation trains could induce cross‐modal adaptation of gustatory events, within cortical regions identified as responsive for both sensory modalities. Within the regions of the insula exhibiting co‐activation for gustation and interoception, a series of paired t‐tests was performed to compare Taste events within the GIA task that followed Interoception and Exteroception adaptation trains. For example, SWEET events that occurred after Interoception adaptation trains (SWEETIA) were compared to SWEET events that occurred after Exteroception adaptation trains (SWEETEA). Importantly, at no point were responses during Taste events compared to responses during Interoception or Exteroception events. Only identical events were compared. As a result, the only difference between SWEETIA and SWEETEA stimuli was the context set by the adaptation trains that preceded those events.
The results of these ROI analyses were used to establish whether the identified interoception‐specific ROIs contained neuronal populations with a shared responsiveness for interoceptive and gustatory processing. This was determined if the ROI met the following criteria: (A) the response to SWEETIA events was significantly less than the response to SWEETEA events, indicating that the gustatory response within those voxels was significantly adapted by interoceptive attention; and (B) the response to NEUTRALIA events was NOT significantly different from the response to NEUTRALEA events, indicating that the adaptation response was specific to the taste of sucrose and did not reflect adaptation of any shared components of both tastant events, such as oral somatosensation.
Finally, in order to examine whether participants body mass affected any cortical adaptation responses, we conducted a series of ROI analyses examining the correlation between body‐mass‐index (BMI) and adaptation magnitude for Interoceptive events (I A − I B), Exteroceptive events (E A − E B), and Sweet (SWEETEA – SWEETIA), and Neutral tastants (NEUTRALEA – NEUTRALIA).
RESULTS
In the group analyses of the GIA task imaging data, we identified multiple regions on the cortical surface that exhibited interoception‐specific adaptation, defined as a significant adaptation response to interoception and not to exteroception trains (Fig. 3, Tables 1 and 2). The brain regions identified as interoception‐specific included bilateral regions of dorsal mid‐insula (Fig. 3, Table 2). These dorsal mid‐insula clusters were located in the region of the posterior short insular gyri and central insular sulcus, a location previously identified as (A) part of primary gustatory cortex [Iannilli et al., 2014; Ogawa et al., 2005; Veldhuizen et al., 2011], (B) selective for interoceptive attention to visceral sensations [Simmons et al., 2013a], and (C) co‐activated by interoceptive and gustatory tasks [Avery et al., 2015]. Interoception‐specific adaptation was also observed in a region of left ventral insula and the bilateral dorsal anterior cingulate cortex [Fig. 3, Table 2].
Using imaging data acquired during the Gustatory Mapping task (GM), which subjects performed immediately prior to the GIA task (see Methods section for details), we next tested whether those interoception‐specific regions of the brain were also responsive to gustatory stimulation. Within each of these five regions‐of‐interest, we performed a series of paired‐sample t‐tests to identify whether they exhibited a reliably greater response to sweet vs. neutral tastants during the GM task. All three identified regions of the insula met this criterion (t (14); P < 0.05; Fig. 4, Table 3). On the contrary, the anterior cingulate regions did not exhibit a significant positive response to either tastant, nor did the activation of these regions discriminate between the two tastants (t (14); P < 0.05).
To evaluate between the Labeled Line and Sensory Convergence models, we next assessed whether those regions of the insula—identified as interoceptive and gustatory responsive—also exhibited cross‐modal adaptation of tastant responses by interoception. To do this, we compared tastant events that followed the interoception adaptation trains to identical tastant events that followed the exteroception adaptation trains. Both dorsal mid‐insula regions exhibited cross‐modal adaptation of tastant responses by interoception, as the response to sweet tastant events following interoception trains was significantly less than the un‐adapted response to sweet tastant events following exteroception trains (left: t (14) = 2.56, P < 0.02; right: t (14) = 2.4, P < 0.03; Table 4, Fig. 5). In contrast, the ventral insula region did not exhibit an adapted response to sweet tastants by interoception (P = 0.1; Table 4). Importantly, within the dorsal mid‐insula, this cross‐modal interoceptive adaptation was specific to the taste of sucrose, as the response to neutral tasteless solutions that followed either interoception or exteroception trains did not differ (P > 0.25; Table 4). We observed no significant relationships between adaptation magnitude and BMI (Table 5).
Table 4.
Adaptation of tastant events within brain regions co‐activated by gustation and interoception
| Exteroceptive adaptation (EA) | Interoceptive adaptation (IA) | EA − IAa | ||||||
|---|---|---|---|---|---|---|---|---|
| % Signal change | % Signal change | % Signal change | ||||||
| Mean | SD | P | Mean | SD | P | t (14) | P | |
| Sweet event | ||||||||
| L dorsal mid‐insula | 0.55 | 0.25 | <0.001 | 0.42 | 0.31 | <0.001 | 2.56 | 0.02 |
| L ventral insula | 0.46 | 0.29 | <0.001 | 0.35 | 0.24 | <0.001 | 1.77 | 0.10 |
| R dorsal mid‐insula | 0.51 | 0.31 | <0.001 | 0.36 | 0.28 | <0.001 | 2.43 | 0.03 |
| Neutral event | ||||||||
| L dorsal mid‐insula | 0.55 | 0.36 | <0.001 | 0.48 | 0.36 | <0.001 | 1.17 | 0.26 |
| L ventral insula | 0.47 | 0.36 | <0.001 | 0.44 | 0.40 | <0.001 | 0.28 | 0.78 |
| R dorsal mid‐insula | 0.60 | 0.37 | <0.001 | 0.50 | 0.42 | <0.001 | 1.15 | 0.27 |
t values were computed using two‐tailed paired t‐tests.
A, anterior; P, posterior.
Figure 5.
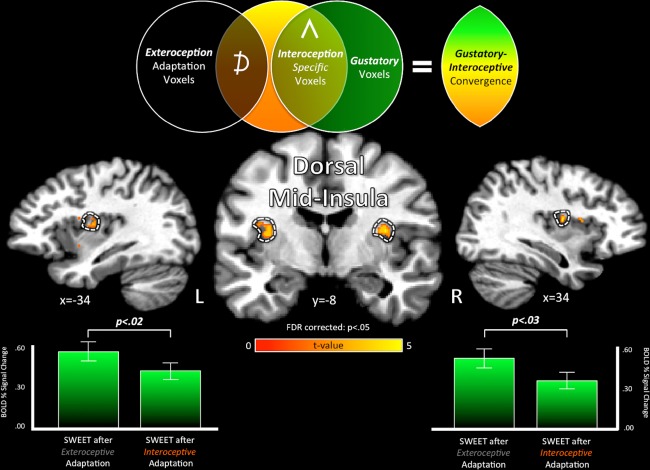
Gustatory‐interoceptive convergence in the dorsal mid‐insula. The bilateral dorsal mid‐insula exhibited specific adaptation for interoception attention (Fig. 3; pictured here in volumetric cortical images) as well as significant activation to the taste of sucrose (Fig. 4). These regions also exhibited an adapted response to sweet tastants following Interoception adaptation trains compared to identical sweet tastants following Exteroception adaptation trains (SWEET after Exteroception—SWEET after interoception, t (14), two‐tailed paired t‐test; Table 4). This demonstrates that the dorsal mid‐insula contains a population of multimodal neurons that respond to both interoceptive and gustatory signals. Additionally, this gustatory adaptation by interoception was specific to the sweet tastant (0.6 M sucrose) and not the neutral tastant (distilled water) (Table 4; not pictured). The specificity of this interoceptive adaptation effect to energy‐rich gustatory stimuli suggests that these multimodal neurons may be involved in the homeostatic maintenance of energy intake. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 5.
Relationships between adaptation magnitude and body mass index (BMI)
| Adaptation magnitude × BMIa | ||||
|---|---|---|---|---|
| Region of interest | Sweet (IA − EA) | Neutral (IA − EA) | I A − I B | E A − E B |
| L dorsal mid‐insula | −0.26 | −0.25 | −0.19 | −0.26 |
| L ventral insula | −0.25 | 0.02 | 0.02 | 0.20 |
| L dorsal anterior insula | −0.32 | −0.10 | −0.03 | 0.10 |
| R dorsal mid‐insula | −0.24 | −0.25 | −0.07 | 0.01 |
| R dorsal anterior insula | −0.12 | −0.11 | 0.19 | 0.18 |
| R anterior cingulate cortex | −0.05 | −0.26 | −0.12 | −0.27 |
| L anterior cingulate cortex | 0.24 | 0.36 | −0.08 | −0.25 |
| L medial frontal gyrus | −0.11 | −0.09 | 0.15 | −0.29 |
r(13), pearson product‐moment coefficient.
DISCUSSION
Prior experimental evidence that the human mid‐insular cortex supports a shared representation of gustatory and interoceptive information [Avery et al., 2015] suggests the possibility that this shared responsiveness extends to the level of individual neurons as well, an idea that finds support in translational research in rodent insular cortex [Hanamori et al., 1998b]. In order to explore this possibility within the human insula, we designed an fMRI‐Adaptation paradigm [Grill‐Spector and Malach, 2001] to examine whether neuronal adaptation induced by repetition of interoceptive stimuli would also adapt gustatory responses within specific regions of the insula. In fMRI‐Adaptation paradigms, successive repetitions of identical stimuli will decrease, or adapt, the activity of a population of neurons sensitive to that stimulus, indicated by the decreased response of a fMRI voxel to subsequent repetitions of a stimulus after even a single presentation of that stimulus [Grill‐Spector et al., 2006]. When a nonidentical stimulus is then presented, the activity of that voxel will either remain diminished if that stimulus activates the same population of adapted neurons, or will instead rebound if the novel stimulus activates a different population of neurons within that voxel. For instance, repeated presentations of the same object in images that slightly vary in rotation, size, and illumination has enabled researchers to discriminate specific neuronal populations within human visual cortex that are specifically responsive to those object transformations [Grill‐Spector et al., 1999], findings which have been subsequently verified by single‐neuron recording studies [Sawamura et al., 2006]. Applied to the present study, when we observe changes in MR signal intensity within a gray‐matter voxel evoked by a specific stimulus (i.e. the delivery of a sweet tastant), this represents the activity of a population of neurons located within that voxel. If we then observe significant differences in the gustatory‐evoked MR signal intensity, depending upon whether it is immediately preceded by an interoceptive (vs. exteroceptive) stimulus, a reasonable interpretation is that the gustatory‐responsive neuronal population is also responsive to some functional property of the preceding interoceptive stimulus. If this were not the case, we would observe no difference in MR signal intensity to the gustatory stimulus, regardless of the preceding stimulus.
Within the bilateral dorsal mid‐insula, successive repetitions of an interoceptive attention task induced both significant adaptation of interoceptive responses as well as significant adaptation of gustatory responses by interoception. Notably, the right dorsal mid‐insula ROI, located at the intersection of the central and superior insular sulci (Fig. 3), rested in approximately the same cortical location as a region identified in a previous study that exhibited co‐activation for interoceptive attention to heart, stomach, and bladder sensations, as well as the taste of sucrose [Avery et al., 2015]. Additionally, the hemodynamic response to sweet, but not neutral tastants was adapted within these dorsal mid‐insula regions, indicating that this gustatory‐interoceptive adaptation is specific to gustatory stimulation and does not simply result from oral somatosensory adaptation within the mid‐insula. These results demonstrate the existence of a population of neurons within the human mid‐insula that is multimodally responsive to both gustatory and interoceptive stimuli, which may play a role in integrating metabolically relevant external stimuli with internal representations of the body's current state.
This finding supports a Sensory Convergence model of gustatory‐interoceptive integration, and is the first demonstration of its kind within the primate brain. This convergence of multimodal sensory inputs within the human mid‐insula may reflect the means by which afferent vagally‐mediated gastro‐intestinal signaling represents the post‐ingestive effects of sucrose, independently of taste signals [Oliveira‐Maia et al., 2012]. Indeed, gustatory cortex in the dorsal mid‐insula also responds in a category‐specific manner to visual food cues [Simmons et al., 2003b, 2005; van der Laan et al., 2011], and is involved in both orthonasal and retronasal olfactory perception [Cerf‐Ducastel and Murphy, 2001; Small et al., 2005]. Posterior regions of the insular cortex have also been shown to respond to temperature and nociceptive stimulation [Brooks et al., 2005; Peltz et al., 2011], which also stimulate gustatory neurons in the rodent insula [Hanamori et al., 1998b], suggesting that thermal and nociceptive properties of food may also be integrated within this insula region. These various lines of evidence suggest that this multi‐modal sensory convergence may be the means by which this region supports a modality‐general representation of food percepts from the internal and external environment.
Additionally, this gustatory‐interoceptive convergence could also serve a more direct role in the regulation of feeding behavior. Within the context of this study, direct adaptation of taste signals by interoceptive information may be associated with such behavioral phenomena as satiety‐induced aversion to food, as demonstrated by prior human neuroimaging evidence that rCBF in the mid‐insula decreases along with the decreasing reward value of chocolate, when subjects consume it to satiety [Small et al., 2001]. Similarly, the mid‐insula's response to visual food stimuli is negatively correlated with peripheral blood glucose, a physiological marker of energy availability [Simmons et al., 2013b]. These findings potentially result from a homeostatic maintenance of the brain's response to food signals; which would be alternately increased or diminished, according to the body's current energy requirements. The findings of the present study highlight one mechanism by which this modulation may occur: the integration of modality‐general food representations (e.g., the sight, taste, smell, and/or post‐ingestive effects of food) with interoceptive representations of the body's homeostatic needs within the neurons of the dorsal mid‐insula. This is consistent with the notion that the insular cortex functions as an orosensory integrative system, regulating the detection and behavioral response to nutrients, according to the body's internal state [de Araujo et al., 2012, 2013].
The mid‐insula has also been implicated in the hedonic component of taste perception [Small, 2010], potentially owing to direct neural connections to the amygdala and orbitofrontal cortex [Scott and Plata‐Salaman, 1999]. Through this connectivity to the brain's reward circuitry and its support of multidimensional representations of food stimuli that can be modulated by energy signals from the body, this multisensory convergence within the insular cortex may serve as a critical extra‐hypothalamic mechanism through which homeostatic signals from the body are able to influence consumptive behavior.
Previous functional neuroimaging research has indicated that obese individuals exhibit greater insula activity to gustatory stimuli than healthy weight individuals [Stice et al., 2008]. Furthermore, obesity is also associated with diminished sensitivity to cardiac signals during heartbeat detection tasks [Kleckner et al., 2015; Rouse et al., 1988]. The disordered feeding present in obesity partially results from a diminished homeostatic balance, which may result from the inability to integrate interoceptive satiety signals to serve as a check upon eating behavior. Within the present study, we observed no relationship between body mass index and magnitude of gustatory adaptation. However, future studies examining relationships between interoceptive sensitivity, interoceptive adaptation, and obesity may be able to further explore this possibility.
In the present study, a heartbeat attention task was used as the specific interoceptive stimulus, in order to avoid potential concerns about the shared semantic content of the visually‐presented text stimuli. Evidence from a previous study indicates that gustatory and interoceptive activations overlap in the same region of the insula, regardless of modality [see Avery et al. 2015, Supporting Information Fig. S3]. The present study provides evidence that those overlapping representations are due to the activation of the same neuronal population within the dorsal mid‐insula. This provides a possible mechanism for the homeostatic integration of interoceptive and gustatory signals within the insula. But as these adaptation effects were observed using a heartbeat interoception condition, it does not provide specific evidence of the convergence of gustatory and feeding‐related interoceptive signals.
One potential explanation for these results is that this gustatory‐interoceptive convergence within the mid‐insula occurs along multiple channels of interoceptive information (i.e. cardiac, respiratory, gastro‐intestinal), but with preferential weight given to feeding‐related interoceptive signals. Electrophysiology studies of neurons within the rodent insular cortex, where this gustatory‐interoceptive convergence was also observed, identified that these neurons are responsive both to multiple tastants (sweet, salty, bitter, and sour) as well as the stimulation of multiple types of nociceptive and interoceptive stimuli [Hanamori et al., 1998b]. This would suggest a broader set of possible functions for this gustatory‐interoceptive convergence than merely that of optimizing glucose availability in the bloodstream. For instance, signals from arterial baroreceptors could modify the neural response to salty stimuli to compensate for elevated or decreased blood pressure. This mechanism could also play a pivotal role in addiction, as substance use behaviors such as cigarette smoking have both a gustatory an interoceptive component. During abstinence states, the interoceptive signals of nicotine withdrawal from the body, processed within the insula, may interact with the gustatory and visceral sensations associated with cigarette smoking, also represented in the insula, in order to modulate the pleasure associated with smoking a cigarette [Avery et al., 2017; Naqvi and Bechara, 2010; Paulus et al., 2009].
The clinical relevance of the present study is additionally underscored by recent evidence that individuals with major depressive disorder (MDD) and anorexia nervosa exhibit abnormally decreased responses to interoceptive attention within the dorsal mid‐insula [Avery et al., 2014; Kerr et al., 2016]. The disruption of visceral interoceptive processing in the mid‐insula may thus hamper the ability of this cortical region to integrate gustatory stimuli with interoceptive information from the body, leading to a disruption of the normal homeostatic maintenance of feeding behavior. This may then partially underlie the abnormal dorsal mid‐insula responsiveness to food pictures in subjects exhibiting depression‐related decreases in appetite [Simmons et al., 2016]. Taken together, these results suggest that abnormal feeding behavior in MDD and eating disorders goes hand‐in‐hand with abnormal interoception as common symptoms of dysregulated insula function [Kaye et al., 2009; Paulus and Stein, 2010].
Limitations
The interoceptive attention condition was designed to take advantage of the attentional spotlight effect, a phenomenon in which attention to a sensory modality will amplify the neural activity within regions of that modality's primary sensory cortex [Johansen‐Berg et al., 2000; Somers et al., 1999; Veldhuizen et al., 2007]. Previous studies employing this task [Avery et al., 2015; Simmons et al., 2013a], along with the present one, identified a region of the dorsal mid‐insula that lies in the approximate location of the terminus of the vagal afferent pathway that relays viscerosensory signals to the cortex through the brainstem and thalamus [Craig, 2002; Pritchard et al., 1986]. This region of the insula has been shown to be responsive to viscerosensory stimulation in rodent and monkey electrophysiology studies [Hanamori et al., 1998a; Zhang et al., 1998], as well as human neuroimaging studies [Cameron, 2002; Wang et al., 2008]. What these various studies suggest is that, while attention to a sensory modality and direct stimulation of that sensory modality are qualitatively different phenomena, they both result in the activation of the same underlying cortical neurocircuitry. However, while the present study does provide evidence for gustatory‐interoceptive convergence within the human insula, these results are only directly applicable within the context of this study, i.e. gustatory adaptation by visceral interoceptive attention. Future studies employing such interoceptive manipulations as gastric distension or isoproterenol infusion (both of which also activate the dorsal mid‐insula [Cameron, 2002; Wang et al., 2008]) might provide further empirical evidence for this model of gustatory‐interoceptive convergence.
CONCLUSIONS
The dorsal mid‐insula supports a joint cortical representation for tasks involving visceral interoceptive attention as well as taste processing. The application of fMRI‐Adaptation methods to examine the functional characteristics of the neurons within this area strongly indicate that the dorsal mid‐insula contains a population of neurons specifically receptive to both gustatory and interoceptive stimuli. Though neurons with these particular response properties have previously been identified in rodent insular cortex [Hanamori et al., 1998b], this is the first demonstration of its kind within a primate brain. The identification of a population of neurons within the dorsal mid‐insula with a shared responsiveness for interoception and gustation suggests that this region may be involved in the integration of visceral and gustatory signals, which could potentially serve to modulate feeding behavior in service of homeostasis.
ACKNOWLEDGMENTS
The authors thank Joel Barcalow and Jennifer Dobson for their help with subject recruitment and assessment.
REFERENCES
- Argall BD, Saad ZS, Beauchamp MS (2006): Simplified intersubject averaging on the cortical surface using SUMA. Hum Brain Mapp 27:14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery JA, Burrows K, Kerr KL, Bodurka J, Khalsa S, Paulus MP, Simmons WK (2017): How the brain wants what the body needs: The neural basis of positive alliesthesia. Neuropsychopharmacology. 27:14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK (2014): Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol Psychiatry 76:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery JA, Kerr KL, Ingeholm JE, Burrows K, Bodurka J, Simmons WK (2015): A common gustatory and interoceptive representation in the human mid‐insula. Hum Brain Mapp 36:2996–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL (2000): Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85:1–17. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I (2005): Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage 27:201–209. [DOI] [PubMed] [Google Scholar]
- Cameron OG (2002): Regional brain activation due to pharmacologically induced adrenergic interoceptive stimulation in humans. Psychosom Med 64:851–861. [DOI] [PubMed] [Google Scholar]
- Cerf‐Ducastel B, Murphy C (2001): fMRI activation in response to odorants orally delivered in aqueous solutions. Chem Senses 26:625–637. [DOI] [PubMed] [Google Scholar]
- Craig AD (2002): How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 33:655–666. [DOI] [PubMed] [Google Scholar]
- Dale AM (1999): Optimal experimental design for event‐related fMRI. Hum Brain Mapp 8:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Geha P, Small DM (2012): Orosensory and homeostatic functions of the insular taste cortex. Chemosens Percept 5:64–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Lin T, Veldhuizen MG, Small DM (2013): Metabolic regulation of brain response to food cues. Curr Biol 23:878–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts SJ, Chow CC, Martin A (2012): Repetition priming and repetition suppression: A case for enhanced efficiency through neural synchronization. Cogn Neurosci 3:227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill‐Spector K, Henson R, Martin A (2006): Repetition and the brain: Neural models of stimulus‐specific effects. Trends Cogn Sci 10:14–23. [DOI] [PubMed] [Google Scholar]
- Grill‐Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R (1999): Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron 24:187–203. [DOI] [PubMed] [Google Scholar]
- Grill‐Spector K, Malach R (2001): fMR‐adaptation: A tool for studying the functional properties of human cortical neurons. Acta Psychol (Amst) 107:293–321. [DOI] [PubMed] [Google Scholar]
- Hanamori T, Kunitake T, Kato K, Kannan H (1998a): Neurons in the posterior insular cortex are responsive to gustatory stimulation of the pharyngolarynx, baroreceptor and chemoreceptor stimulation, and tail pinch in rats. Brain Res 785:97–106. [DOI] [PubMed] [Google Scholar]
- Hanamori T, Kunitake T, Kato K, Kannan H (1998b): Responses of neurons in the insular cortex to gustatory, visceral, and nociceptive stimuli in rats. J Neurophysiol 79:2535–2545. [DOI] [PubMed] [Google Scholar]
- Iannilli E, Noennig N, Hummel T, Schoenfeld AM (2014): Spatio‐temporal correlates of taste processing in the human primary gustatory cortex. Neuroscience 273:92–99. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H, Christensen V, Woolrich M, Matthews PM (2000): Attention to touch modulates activity in both primary and secondary somatosensory areas. Neuroreport 11:1237–1241. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Fudge JL, Paulus M (2009): New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci 10:573–584. [DOI] [PubMed] [Google Scholar]
- Kerr KL, Moseman SE, Avery JA, Bodurka J, Zucker NL, Simmons WK (2016): Altered insula activity during visceral interoception in weight‐restored patients with anorexia nervosa. Neuropsychopharmacology 41:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner IR, Wormwood JB, Simmons WK, Barrett LF, Quigley KS (2015): Methodological recommendations for a heartbeat detection‐based measure of interoceptive sensitivity. Psychophysiology 52:1432–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A (2010): The insula and drug addiction: An interoceptive view of pleasure, urges, and decision‐making. Brain Struct Funct 214:435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H, Wakita M, Hasegawa K, Kobayakawa T, Sakai N, Hirai T, Yamashita Y, Saito S (2005): Functional MRI detection of activation in the primary gustatory cortices in humans. Chem Senses 30:583–592. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Wang XD (2002): Neurons in the cortical taste area receive nociceptive inputs from the whole body as well as the oral cavity in the rat. Neurosci Lett 322:87–90. [DOI] [PubMed] [Google Scholar]
- Oliveira‐Maia AJ, de Araujo IE, Monteiro C, Workman V, Galhardo V, Nicolelis MA (2012): The insular cortex controls food preferences independently of taste receptor signaling. Front Syst Neurosci 6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB (2010): Interoception in anxiety and depression. Brain Struct Funct 214:451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schulteis G (2009): The role of interoception and alliesthesia in addiction. Pharmacol Biochem Behav 94:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz E, Seifert F, DeCol R, Dorfler A, Schwab S, Maihofner C (2011): Functional connectivity of the human insular cortex during noxious and innocuous thermal stimulation. Neuroimage 54:1324–1335. [DOI] [PubMed] [Google Scholar]
- Pritchard TC, Hamilton RB, Morse JR, Norgren R (1986): Projections of thalamic gustatory and lingual areas in the monkey, Macaca fascicularis. J Comp Neurol 244:213–228. [DOI] [PubMed] [Google Scholar]
- Rouse CH, Jones GE, Jones KR (1988): The effect of body composition and gender on cardiac awareness. Psychophysiology 25:400–407. [DOI] [PubMed] [Google Scholar]
- Sawamura H, Orban GA, Vogels R (2006): Selectivity of neuronal adaptation does not match response selectivity: A single‐cell study of the FMRI adaptation paradigm. Neuron 49:307–318. [DOI] [PubMed] [Google Scholar]
- Scott TR, Plata‐Salaman CR (1999): Taste in the monkey cortex. Physiol Behav 67:489–511. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P (2013a): Keeping the body in mind: Insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum Brain Mapp 34:2944–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Burrows K, Avery JA, Kerr KL, Bodurka J, Savage CR, Drevets WC (2016): Depression‐related increases and decreases in appetite: dissociable patterns of aberrant activity in reward and interoceptive neurocircuitry. Am J Psychiatry 173:418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Martin A, Barsalou LW (2005): Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb Cortex 15:1602–1608. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Rapuano KM, Kallman SJ, Ingeholm JE, Miller B, Gotts SJ, Avery JA, Hall KD, Martin A (2013b): Category‐specific integration of homeostatic signals in caudal but not rostral human insula. Nat Neurosci 16:1551–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM (2010): Taste representation in the human insula. Brain Struct Funct 214:551–561. [DOI] [PubMed] [Google Scholar]
- Small DM, Gerber JC, Mak YE, Hummel T (2005): Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron 47:593–605. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones‐Gotman M (2001): Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain 124:1720–1733. [DOI] [PubMed] [Google Scholar]
- Somers DC, Dale AM, Seiffert AE, Tootell RB (1999): Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proc Natl Acad Sci USA 96:1663–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM (2008): Relation of reward from food intake and anticipated food intake to obesity: A functional magnetic resonance imaging study. J Abnorm Psychol 117:924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan LN, de Ridder DT, Viergever MA, Smeets PA (2011): The first taste is always with the eyes: A meta‐analysis on the neural correlates of processing visual food cues. Neuroimage 55:296–303. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA (1997): Structural and functional analyses of human cerebral cortex using a surface‐based atlas. J Neurosci 17:7079–7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen MG, Albrecht J, Zelano C, Boesveldt S, Breslin P, Lundstrom JN (2011): Identification of human gustatory cortex by activation likelihood estimation. Hum Brain Mapp 32:2256–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen MG, Bender G, Constable RT, Small DM (2007): Trying to detect taste in a tasteless solution: Modulation of early gustatory cortex by attention to taste. Chem Senses 32:569–581. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Tomasi D, Backus W, Wang R, Telang F, Geliebter A, Korner J, Bauman A, Fowler JS, Thanos PK, Volkow ND (2008): Gastric distention activates satiety circuitry in the human brain. Neuroimage 39:1824–1831. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Dougherty PM, Oppenheimer SM (1998): Characterization of baroreceptor‐related neurons in the monkey insular cortex. Brain Res 796:303–306. [DOI] [PubMed] [Google Scholar]


