Figure 2. Mpa has a unique β-grasp-fold domain near the C-terminus.
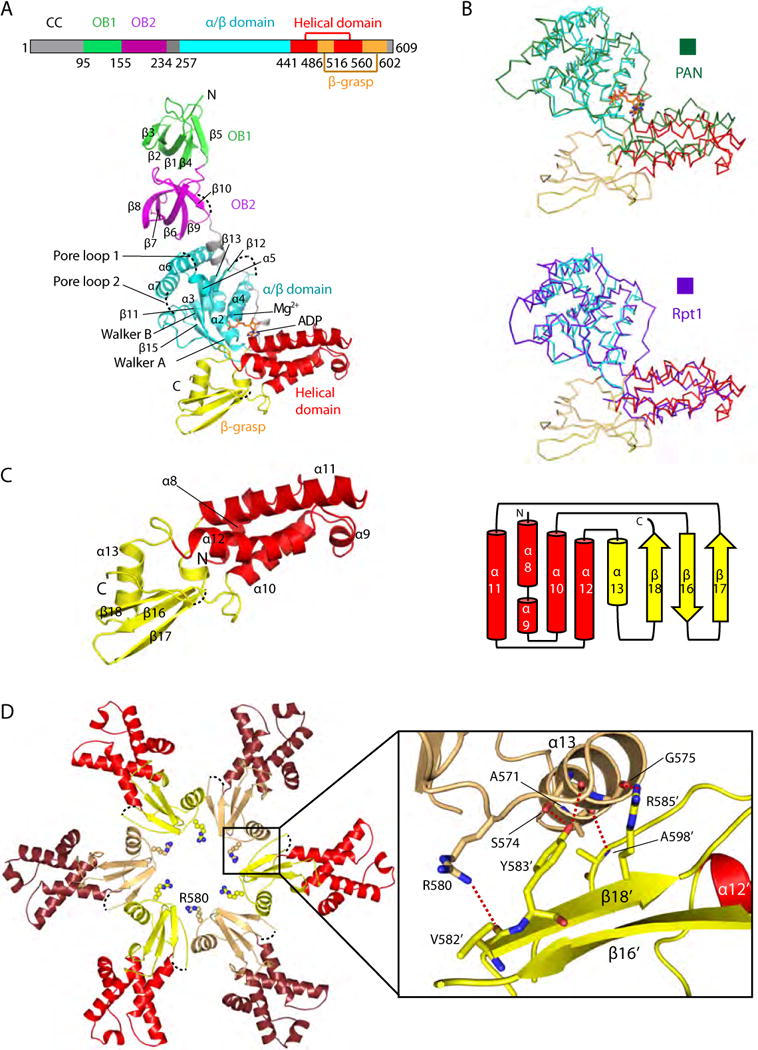
(A) Top: Domain mapof Mpa. Bottom: Structure of a singleMpaprotomer, with the OB1 domain in green, OB2 in purple, the large AAA subdomain in cyan, the small AAA subdomain in red, and the β-grasp-like domain in yellow. The secondary structure elements are labeled in the OB and ATPase regions. ADP bound to the hinge region between the two AAA subdomains is shown in stick form. Four disordered loops, including pore loop 1 (the Ar-Φloop) and pore loop 2, are shown as dashed black curves. (B) Superimposition of the ATPase region of Mpa with that of PAN (top panel, dark green ribbon) or of the Rpt1 (bottom panel, purple ribbon). The ATPase domains of Mpa, PAN, and Rpt1 are similar, but the C-terminal β-grasp fold is found only in Mpa. (C) Left, structure of the C-terminal β-grasp domain of Mpa; right, topology diagram. (D) The insertion domains interact with each other in anMpa hexamer to form the exit port lined by Arg-580. Five hydrogen bonds at the interface between neighboring β-grasp domains are shown as dashed red lines in the inset window.
