Abstract
BACKGROUND
Hysterectomy is among the most common major surgical procedures performed in women. Approximately 450,000 hysterectomy procedures are performed each year in the United States for benign indications. However, little is known regarding contemporary US hysterectomy trends for women with benign disease with respect to operative technique and perioperative complications, and the association between these 2 factors with patient, surgeon, and hospital characteristics.
OBJECTIVE
We sought to describe contemporary hysterectomy trends and explore associations between patient, surgeon, and hospital characteristics with surgical approach and perioperative complications.
STUDY DESIGN
Hysterectomies performed for benign indications by general gynecologists from July 2012 through September 2014 were analyzed in the all-payer Maryland Health Services Cost Review Commission database. We excluded hysterectomies performed by gynecologic oncologists, reproductive endocrinologists, and female pelvic medicine and reconstructive surgeons. We included both open hysterectomies and those performed by minimally invasive surgery, which included vaginal hysterectomies. Perioperative complications were defined using the Agency for Healthcare Research and Quality patient safety indicators. Surgeon hysterectomy volume during the 2-year study period was analyzed (0–5 cases annually = very low, 6–10 = low, 11–20 = medium, and ≥21 = high). We utilized logistic regression and negative binomial regression to identify patient, surgeon, and hospital characteristics associated with minimally invasive surgery utilization and perioperative complications, respectively.
RESULTS
A total of 5660 hospitalizations were identified during the study period. Most patients (61.5%) had an open hysterectomy; 38.5% underwent a minimally invasive surgery procedure (25.1% robotic, 46.6% laparoscopic, 28.3% vaginal). Most surgeons (68.2%) were very low– or low-volume surgeons. Factors associated with a lower likelihood of undergoing minimally invasive surgery included older patient age (reference 45–64 years; 20–44 years: adjusted odds ratio, 1.16; 95% confidence interval, 1.05–1.28), black race (reference white; adjusted odds ratio, 0.70; 95% confidence interval, 0.63–0.78), Hispanic ethnicity (adjusted odds ratio, 0.62; 95% confidence interval, 0.48–0.80), smaller hospital (reference large; small: adjusted odds ratio, 0.26; 95% confidence interval, 0.15–0.45; medium: adjusted odds ratio, 0.87; 95% confidence interval, 0.79–0.96), medium hospital hysterectomy volume (reference ≥200 hysterectomies; 100–200: adjusted odds ratio, 0.78; 95% confidence interval, 0.71–0.87), and medium vs high surgeon volume (reference high; medium: adjusted odds ratio, 0.87; 95% confidence interval, 0.78–0.97). Complications occurred in 25.8% of open and 8.2% of minimally invasive hysterectomies (P < .0001). Minimally invasive hysterectomy (adjusted odds ratio, 0.22; 95% confidence interval, 0.17–0.27) and large hysterectomy volume hospitals (reference ≥200 hysterectomies; 1–100: adjusted odds ratio, 2.26; 95% confidence interval, 1.60–3.20; 101–200: adjusted odds ratio, 1.63; 95% confidence interval, 1.23–2.16) were associated with fewer complications, while patient payer, including Medicare (reference private; adjusted odds ratio, 1.86; 95% confidence interval, 1.33–2.61), Medicaid (adjusted odds ratio, 1.63; 95% confidence interval, 1.30–2.04), and self-pay status (adjusted odds ratio, 2.41; 95% confidence interval, 1.40–4.12), and very-low and low surgeon hysterectomy volume (reference ≥21 cases; 1–5 cases: adjusted odds ratio, 1.73; 95% confidence interval, 1.22–2.47; 6–10 cases: adjusted odds ratio, 1.60; 95% confidence interval, 1.11–2.23) were associated with perioperative complications.
CONCLUSION
Use of minimally invasive hysterectomy for benign indications remains variable, with most patients undergoing open, more morbid procedures. Older and black patients and smaller hospitals are associated with open hysterectomy. Patient race and payer status, hysterectomy approach, and surgeon volume were associated with perioperative complications. Hysterectomies performed for benign indications by high-volume surgeons or by minimally invasive techniques may represent an opportunity to reduce preventable harm.
Keywords: benign indication, complications, hysterectomy, minimally-invasive, surgeon volume
Introduction
Hysterectomy is among the most common major surgical procedures performed in women. Approximately 450,000 hysterectomy procedures are performed each year in the United States.1–3 The majority of cases are performed for benign gynecologic conditions, including uterine leiomyomata, endometriosis, abnormal menstrual bleeding, and pelvic organ prolapse.1,2
Compared with laparotomy (ie, open surgery), utilization of minimally invasive surgery (MIS), which comprises vaginal, laparoscopic, or robotic-assisted procedures, decreases perioperative complications, improves patient quality of life, and lowers health care costs for cologic conditions.4–13 A Cochrane Review examining 47 randomized controlled trials demonstrated that minimally invasive hysterectomy performed for benign indications resulted in a faster return to normal activities for patients relative to abdominal hysterectomies.14 Additionally, a 2007 through 2011 nationwide study determined that, for women undergoing hysterectomy for nonmetastatic endometrial cancer, open hysterectomy was associated with a higher risk of surgical site infections, venous thromboembolism, and prolonged hospital stay.15 This same analysis demonstrated that MIS hysterectomy utilization across the United States was low overall and varied considerably by patient and hospital factors, suggesting a disparity in the quality of gynecologic surgical care delivered nationwide.15
However, little is known regarding contemporary US hysterectomy trends for women with benign disease. Given that most national surgical databases include hospital-level, but not surgeon-level, data, even less is known about surgeon characteristics associated with hysterectomy approach and perioperative complications after hysterectomy– both important metrics in gynecologic surgery quality improvement initiatives. This study examines hysterectomy trends within a statewide, all-payer registry: the Maryland Health Services Cost Review Commission (HSCRC) database. The HSCRC database captures all surgical encounters performed in regulated spaces within the state and provides the data points necessary for exploring surgical trends and the impact of patient-, hospital-, and surgeon-level factors on procedural utilization and outcomes. We hypothesized that MIS hysterectomy utilization for benign gynecologic conditions varies considerably and that perioperative complications are related to hysterectomy approach, by patient, surgeon, and hospital factors.
Materials and Methods
The institutional review board of the Johns Hopkins School of Medicine, Baltimore, MD, found this as an exempt study.
Maryland HSCRC database
We utilized Maryland’s all-payer claims database, the HSCRC, to identify all hysterectomies performed for apparent benign disease in the state’s 62 hospitals from July 1, 2012, through Sept. 30, 2014. Of note, this database captures patients who undergo same-day surgery and hospital discharge as well as those patients admitted to the hospital for ≥23 hours. Eligible procedures were those performed by physicians identified within the American Medical Association master file with a primary specialty of obstetrics and gynecology (OB/GYN). Hysterectomies performed by general surgeons and sub-specialty gynecologic surgeons (ie, gynecologic oncologists, reproductive endocrinologists, and female pelvic medicine and reconstructive surgeons) were excluded, as these physicians were more likely to perform concurrent procedures with hysterectomy that could potentially confound the analysis.
Hysterectomy was defined using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure codes. Open abdominal (68.3, 68.39, 68.4, 68.49) and minimally invasive procedures were eligible. Minimally invasive procedures included laparoscopic hysterectomy (68.31, 68.41, 68.51), vaginal hysterectomy (68.5, 68.59), and robotic-assisted procedures (17.4, 17.41, 17.42). Benign indications included benign neoplasm or cyst (215.6, 219, 220, 221, 620.0, 620.1, 620.2, 621.0, 752), fibroids (218), endometriosis (617), pelvic organ prolapse (618), and abnormal menstrual bleeding (626, 627). Individuals who had codes associated with obstetrical procedures (72, 73, 74) or malignant gynecologic, urologic, or abdominopelvic neoplasms were excluded (179, 180, 181, 182, 183, 184, 188). We also excluded patients who had urologic or vaginal conditions as their principal procedure (59.3–5, 59.7, 59.71, 59.79), and patients who had emergency department charges, suggesting that these hysterectomies were not elective.
Age, race, Elixhauser comorbidity index, payer status, and surgical indication were considered patient demographic variables. The comorbidities identified by the Elixhauser index are significantly associated with in-hospital mortality and include both acute and chronic conditions; the value of the index indicates the number of comorbidities present.16,17
Surgeon and hospital characteristics
Every surgeon carries a unique National Provider Identifier, which is provided through the Centers for Medicare and Medicaid Services (CMS). To obtain surgeon and select hospital characteristics for each hospitalization in our study, we linked National Provider Identifier numbers to publically available data made available through CMS. Surgeon demographics, including gender and number of years in practice, were obtained. We analyzed surgeon hysterectomy volume during this 2-year study period in groups (0–5 cases annually = very low, 6–10 = low, 11–20 medium, and ≥21 = high). These categories were defined after examining the distribution of surgeon volume in the data set.
We retrieved information on hospital size (small = <100, medium = 100–399, large = ≥400 beds), hospital location, and teaching status from the American Hospital Association. Using the unique hospital identification in our database, we also analyzed hospital hysterectomy volume in groups (1–100 = low, 101–200 = medium, and ≥201 = high).
Incidence of perioperative complications
The Agency for Healthcare Research and Quality has created a list of perioperative complications called patient safety indicators that may potentially occur during a hospitalization.16,17 In this study, the following patient safety indicators were assessed: hemorrhage or hematoma, respiratory failure, pulmonary embolism or deep vein thrombosis, and sepsis. Surgical site infections were identified using the following procedure and diagnostic codes18: 86.01, 83.49, 86.22, 86.28, 86.04, 86.09, 96.59, 91.72–3, 320, 324, 567, 614.3, 682, 711.06, 730.0, 959.9, 996.6, and 998. For pneumonia, we used the following ICD-9-CM diagnosis codes: 480, 481, 482, 483, 484, 485, and 486. Length of stay was obtained from the variable in the HSCRC database. We used the discharge disposition code to determine vital status.
Statistical analysis
Data analysis was computed in software (Stata, Version 13.1; StataCorp, College Station, TX). We used χ2 tests to determine unadjusted P values. Adjusted P values were used when comparing each patient, surgeon, or hospital category by procedure type and to evaluate total perioperative outcomes by procedure. We utilized negative binomial regression when identifying patient, surgeon, and hospital characteristics associated with MIS utilization, to account for outcome overdispersion.19 Multivariable logistic regression identified patient, surgeon, and hospital characteristics associated with perioperative complications. For both regressions, the following factors were adjusted for: surgeon volume, surgeon practicing year, hospital bed size, patient age, patient race, payer, Elixhauser score, and all 5 selected benign indications. In addition, the regression that assessed predictors of complications also adjusted for operative technique (MIS vs open). Finally, we assessed the proportion of surgeons performing only open hysterectomies among the surgeon volume groups.
Results
After selecting for hysterectomy procedures performed by general OB/GYNs and excluding patients with the aforementioned malignant, urologic, gastrointestinal, and vaginal procedures, we were left with our study population of 5660 (Figure 1). Half of the patients (52.0%) were between the ages of 45–64 years, white (42.5%), had an Elixhauser score of 0 or 1 (56.8%), and had commercial/private insurance (71.6%). Indications for hysterectomy varied and were not mutually exclusive (ie, select patients had >1 diagnostic code for their hysterectomy indication). Overall, 65.9% of patients had a hysterectomy indication of fibroids, 57.5% had abnormal menstrual bleeding, 19.2% were diagnosed with a benign neoplasm or ovarian cyst, 16.2% with endometriosis, and 13.6% with pelvic organ prolapse.
FIGURE 1. Inclusion and exclusion criteria for study population.
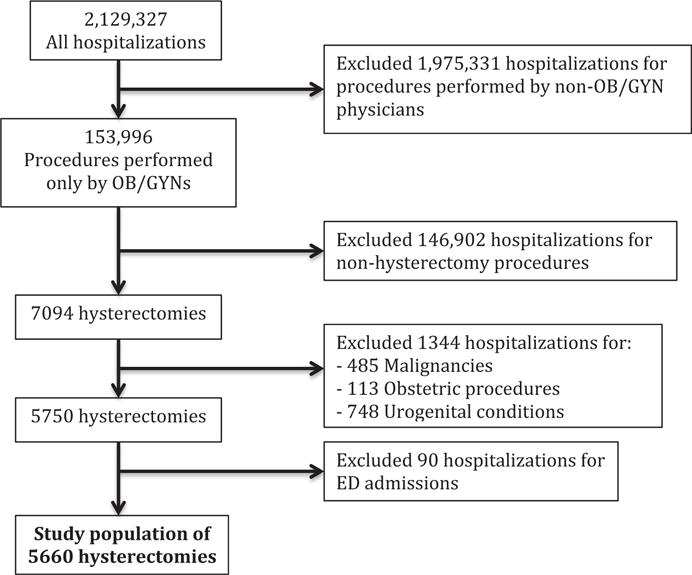
We identified 5660 patients that met our inclusion and exclusion criteria.
ED, emergency department; OB/GYN, obstetrics and gynecology.
Mehta et al. Disparities associated with benign hysterectomy approach and complications. Am J Obstet Gynecol 2017.
The majority (61.5%) of the patients underwent an open hysterectomy and the remaining (38.5%) underwent MIS procedure, comprising 25.1% robotic, 46.6% laparoscopic, and 28.3% vaginal approaches (Figure 2). While white patients represented 42.5% of the study population (Table 1), 56.4% underwent MIS hysterectomy, compared with black and Hispanic patients, who comprised 45.5% of all hospitalizations but only 33.9% underwent MIS hysterectomy. Half of all hysterectomies were performed at medium-volume hospitals for hysterectomies (56.9%), followed by high-volume (25.2%) and low-volume (17.9%) hysterectomy hospitals.
FIGURE 2. Procedure variation for hysterectomies.
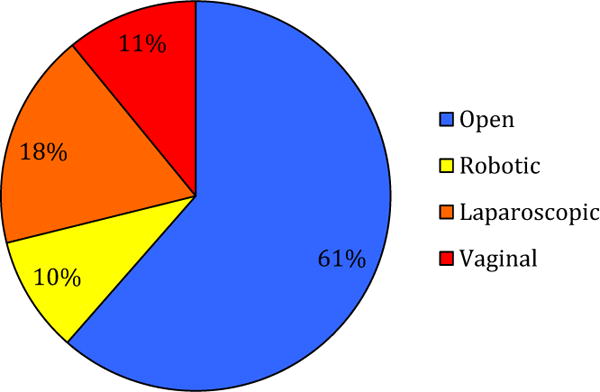
Hysterectomies performed in Maryland from July 2012 through September 2014 had marked variation in operative type. More than half were performed open (61%), followed by laparoscopic (18%), vaginal (11%), and robotic (10%).
Mehta et al. Disparities associated with benign hysterectomy approach and complications. Am J Obstet Gynecol 2017.
TABLE 1.
Patient and hospital characteristics by hysterectomy approach
| All | Open | MIS | Unadjusted P value |
|
|---|---|---|---|---|
| N = 5660 100% |
N = 3478 61.5% |
N = 2182 38.5% |
||
| Race, % | <.01 | |||
| White | 42.5 | 33.7 | 56.4 | |
| Black | 43.7 | 51.8 | 30.9 | |
| Hispanic ethnicity | 1.8 | 4.3 | 3.0 | |
| Other/missing | 10.0 | 10.2 | 9.7 | |
| Age, % | <.01 | |||
| <20 y | 0.02 | 0.0 | 0.1 | |
| 20–44 y | 40.9 | 41.0 | 40.7 | |
| 45–64 y | 52.0 | 55.1 | 47.1 | |
| >65 y | 7.1 | 3.9 | 12.2 | |
| Elixhauser score, % | <.01 | |||
| 0–1 | 56.8 | 54.1 | 61.0 | |
| 2–4 | 40.1 | 42.4 | 36.6 | |
| >5 | 3.1 | 3.5 | 2.3 | |
| Payer, % | <.01 | |||
| Medicare | 9.9 | 7.4 | 13.9 | |
| Medicaid | 14.0 | 15.7 | 11.2 | |
| Self-pay | 1.5 | 1.9 | 1.0 | |
| Commercial/private | 71.6 | 71.9 | 71.2 | |
| Other | 3.0 | 3.1 | 2.7 | |
| Fibroids, % | <.01 | |||
| Yes | 65.9 | 75.9 | 50.0 | |
| No | 34.1 | 24.1 | 50.0 | |
| Endometriosis, % | .06 | |||
| Yes | 16.2 | 15.5 | 17.4 | |
| No | 83.8 | 84.5 | 82.6 | |
| Menstrual bleeding, % | <.01 | |||
| Yes | 57.5 | 60.3 | 53.2 | |
| No | 42.5 | 39.7 | 46.8 | |
| Benign neoplasm/cyst, % | <.01 | |||
| Yes | 19.2 | 21.7 | 15.4 | |
| No | 80.8 | 78.3 | 84.6 | |
| Pelvic organ prolapse, % | <.01 | |||
| Yes | 13.6 | 4.3 | 71.5 | |
| No | 86.4 | 95.7 | 28.5 | |
| Other indication, % | .04 | |||
| Yes | 4.6 | 4.2 | 5.4 | |
| No | 95.4 | 95.8 | 94.6 | |
| Teaching affiliation, % | <.01 | |||
| Teaching | 61.3 | 58.7 | 65.4 | |
| Nonteaching | 38.7 | 41.3 | 34.6 | |
| Hospital location, % | <.01 | |||
| Urban | 96.2 | 95.0 | 98.1 | |
| Rural | 3.8 | 5.0 | 1.9 | |
| Hysterectomy hospital volume, % | <.01 | |||
| Low (< 101) | 17.9 | 19.7 | 15.0 | |
| Medium (101–200) | 56.9 | 57.7 | 55.6 | |
| High (>201) | 25.2 | 22.6 | 29.5 | |
| Hospital bed size, % | <.01 | |||
| Small (<100) | 2.0 | 2.9 | 0.6 | |
| Medium (100–399) | 66.4 | 67.3 | 65.1 | |
| Large (>400) | 31.6 | 29.9 | 34.3 |
MIS, minimally invasive surgery.
Mehta et al. Disparities associated with benign hysterectomy approach and complications. Am J Obstet Gynecol 2017.
A total of 519 surgeons performed at least 1 hysterectomy during the study period (Table 2); most were female (60.1%) and had been in practice >21 years (56.3%). During the approximate 2-year study period, most were very low–volume surgeons (45.1%: 1–5 hysterectomies) or low-volume surgeons (23.1%: 6–10 hysterectomies). Only 14.7% were considered high-volume surgeons (14.7%: ≥21 hysterectomies). Despite this, 47% of patients underwent their procedure at a high hysterectomy–volume hospital (47%), followed by medium hysterectomy– volume hospitals (35%) and low hysterectomy–volume hospitals (18%).
TABLE 2.
Surgeon characteristics
| All surgeons, N = 519 | |
|---|---|
| Gender | |
| Female | 312 (60.1%) |
| Years in practice | |
| ≤20 | 227 (43.7%) |
| ≥21 | 292 (56.3%) |
| Hysterectomy volume | |
| 1–5 = Very low | 234 (45.1%) |
| 6–10 = Low | 120 (23.1%) |
| 11–20 = Medium | 89 (17.2%) |
| ≥21 = High | 76 (14.7%) |
Mehta et al. Disparities associated with benign hysterectomy approach and complications. Am J Obstet Gynecol 2017.
Patients who underwent open hysterectomy surgery were more likely to experience a complication (open: 25.8%, MIS: 8.2%, P <.0001) (Table 3). Specific complication rates by procedure type with unadjusted P values include: length of hospital stay >2 days (open: 25.4%, MIS: 7.3%, P < .0001), surgical site infection (open: 1.3%, MIS: 0.5%, P = .002), postoperative hemorrhage or hematoma (open: 0.8%, MIS: 0.8%, P = .94), postoperative respiratory failure (open: 0.3%, MIS: 0.1%, P = .38), pneumonia (open: 0.2%, MIS: 0.3%, P = .38), postoperative pulmonary embolism or deep vein thrombosis (open: 0.1%, MIS: 0.05%, P = .57), and postoperative sepsis (open: 0.1%, MIS: 0.05%, P = .74).
TABLE 3.
Incidence of selected postoperative complications by procedure
| Complication | Open | MIS | P value |
|---|---|---|---|
| Any complication | 899 (25.8%) | 172 (8.2%) | <.0001a |
| Length of stay >2 d | 882 (25.4%) | 159(7.3%) | <.0001 |
| Surgical site infection | 45 (1.3%) | 10(0.5%) | .002 |
| Postoperative hemorrhage or hematoma | 28 (0.8%) | 18(0.8%) | .94 |
| Postoperative respiratory failure | 9 (0.3%) | 3 (0.1%) | .38 |
| Pneumonia | 7 (0.2%) | 7 (0.3%) | .38 |
| Postoperative PE or DVT | 3 (0.1%) | 1 (0.05%) | .57 |
| Postoperative sepsis | 3 (0.1%) | 1 (0.05%) | .74 |
DVT, deep vein thrombosis; MIS, minimally invasive surgery; PE, pulmonary embolism.
Multivariable regression adjusted for surgeon hysterectomy volume, hospital hysterectomy volume, surgeon practicing year, hospital bed size, patient age, patient race, payer, Elixhauser score, and all 5 selected benign indications.
Mehta et al. Disparities associated with benign hysterectomy approach and complications. Am J Obstet Gynecol 2017.
Younger patient age (reference 45–64 years; 20–44 years: adjusted odds ratio [aOR], 1.16; 95% confidence interval [CI], 1.05–1.28) was favorably associated with MIS utilization, while small (aOR, 0.26; 95% CI, 0.15–0.45) and medium (aOR, 0.87; 95% CI, 0.75–0.96) compared to large hospitals, medium compared to large hysterectomy–volume hospitals (aOR, 0.78; 95% CI, 0.71–0.87), black race (reference white; aOR, 0.70; 95% CI, 0.63–0.78), Hispanic ethnicity (aOR, 0.62; 95% CI, 0.48–0.80), and medium surgeon volume (medium vs high volume: odds ratio, 0.78; 95% CI, 0.71–0.87) were correlated with a lower likelihood of receiving MIS hysterectomy (Table 4). Surgeon years of practice were not associated with MIS utilization (aOR, 0.92; 95% CI, 0.83–1.01). Additionally, a hysterectomy performed for fibroids (aOR, 0.75; 95% CI, 0.68–0.83) and benign neoplasm or cyst (aOR, 0.84; 95% CI, 0.74–0.94) was less likely to be performed minimally invasively, whereas a hysterectomy performed for endometriosis (aOR, 1.14; 95% CI, 1.02–1.28), abnormal menstrual bleeding (aOR, 1.15; 95% CI, 1.05–1.27), or pelvic organ prolapse (aOR, 2.07; 95% CI, 1.82–2.34) was more likely to be performed using MIS approach.
TABLE 4.
Predictors of minimally invasive procedure use by patient, surgeon, and hospital characteristics
| Characteristic | Adjusted odds ratioa (95% CI) |
|---|---|
| Surgeon hysterectomy volume | |
| 1–5 | 1.00(0.87–1.17) |
| 6–10 | 0.92 (0.81–1.05) |
| 11–20 | 0.87 (0.78–0.97) |
| ≥21 | Reference |
| Hospital hysterectomy volume | |
| 1–100 | 0.93 (0.81–1.06) |
| 101–200 | 0.78 (0.71–0.87) |
| ≥201 | Reference |
| Beds | |
| <99 | 0.26 (0.15–0.45) |
| 100–399 | 0.87 (0.79–0.96) |
| ≥400 | Reference |
| Years practicing | |
| <20 | Reference |
| ≥20 | 0.92 (0.83–1.01) |
| Patient age, y | |
| <20 | – |
| 20–44 | 1.16 (1.05–1.28) |
| 45–64 | Reference |
| ≥65 | 1.08 (0.88–1.34) |
| Patient race | |
| White | Reference |
| Black | 0.70 (0.63–0.78) |
| Hispanic ethnicity | 0.62 (0.48–0.80) |
| Other | 0.86 (0.74–1.00) |
| Payer, % | |
| Medicare | 0.99(0.81–1.19) |
| Medicaid | 0.92 (0.80–1.06) |
| Self-pay | 0.79 (0.52–1.21) |
| Commercial/private | Reference |
| Other | 1.02 (0.78–1.32) |
| Elixhauser score | |
| 0–1 | Reference |
| 2–4 | 0.92 (0.84–1.00) |
| ≥5 | 0.82 (0.62–1.10) |
| Fibroids | |
| Yes | 0.75 (0.68–0.83) |
| No | Reference |
| Endometriosis | |
| Yes | 1.14 (1.02–1.28)b |
| No | Reference |
| Abnormal menstruation bleeding | |
| Yes | 1.15 (1.05–1.27)b |
| No | Reference |
| Benign neoplasm or cyst | |
| Yes | 0.84 (0.74—0.94)b |
| No | Reference |
| Pelvic organ prolapse | |
| Yes | 2.07 (1.82–2.34)b |
| No | Reference |
CI, confidence interval.
Multivariable regression adjusted for surgeon hysterectomy volume, hospital hysterectomy volume, hospital bed size, surgeon practicing year, patient age, patient race, payer, Elixhauser score, and all 5 selected benign indications;
Odds ratios and 95% confidence intervals represent significant values.
Mehta et al. Disparities associated with benign hysterectomy approach and complications. Am J Obstet Gynecol 2017.
Several factors were independently associated with perioperative complications. Undergoing MIS hysterectomy procedure was strongly associated with fewer complications (aOR, 0.22; 95% CI, 0.17–0.27) (Table 5). Conversely, patient factors associated with complications included black patient race (reference white; aOR, 1.45; 95% CI, 1.18–1.79), Medicare (reference private insurance; aOR, 1.86; 95% CI, 1.33–2.61) and Medicaid (aOR, 1.63; 95% CI, 1.30–2.04) insurance, no insurance (aOR, 2.41; 95% CI, 1.40–4.12), and comorbidity profile (reference 0–1 comorbidities; 2–4: aOR, 1.67; 95% CI, 1.42–1.97; ≥5: aOR, 4.58; 95% CI, 3.07–6.84). Finally, both surgeon and hospital hysterectomy volume was significantly correlated with complications. Relative to high-volume surgeons performing ≥21 hysterectomies, surgeons performing fewer hysterectomies had higher complication rates (1–5 hysterectomies: aOR, 1.73; 95% CI, 1.22–2.47; 6–10: aOR, 1.60; 95% CI, 1.11–2.23). Relative to high hysterectomy–volume hospitals, low and medium volume–hysterectomy hospitals had higher complication rates (reference ≥201 hysterectomies; 1–100: aOR, 2.26; 95% CI, 1.60–3.20; 101–200: aOR, 1.63; 95% CI, 1.23–2.16). Finally, a statistically greater proportion of very low–, low-, and medium-volume surgeons relative to high-volume surgeons performed only open hysterectomies (33.0% vs 3.9%, P < .001) (Figure 3).
TABLE 5.
Predictors of postoperative complications by patient, surgeon, and hospital characteristics
| Characteristic | Adjusted odds ratiosa (95% CI) |
|---|---|
| Procedure | |
| Open | Reference |
| MIS | 0.22 (0.17–0.27)b |
| Surgeon hysterectomy volume | |
| 1–5 | 1.73 (1.22–2.47)b |
| 6–10 | 1.60 (1.11–2.23)b |
| 11–20 | 1.05 (0.74–1.49) |
| ≥21 | Reference |
| Hospital hysterectomy volume | |
| 1–100 | 2.26 (1.60–3.20)b |
| 101–200 | 1.63 (1.23–2.16)b |
| ≥201 | Reference |
| Beds | |
| <99 | 0.27 (0.11–0.65)b |
| 100–399 | 0.59 (0.45–0.78) |
| ≥400 | Reference |
| Years practicing | |
| <20 | Reference |
| ≥20 | 0.82 (0.63–1.06) |
| Patient age, y | |
| <20 | – |
| 20–44 | 1.09 (0.92–1.30) |
| 45–64 | Reference |
| ≥65 | 1.19(0.77–1.85) |
| Patient race | |
| White | Reference |
| Black | 1.45 (1.18–1.79)b |
| Hispanic ethnicity | 1.54 (0.99–2.38) |
| Other | 1.32 (0.97–1.80) |
| Payer, % | |
| Medicare | 1.86 (1.33–2.61)b |
| Medicaid | 1.63 (1.30–2.04)b |
| Self-pay | 2.41 (1.40–4.12)b |
| Commercial/private | Reference |
| Other | 0.85 (0.52–1.38) |
| Elixhauser score | |
| 0–1 | Reference |
| 2–4 | 1.67 (1.42–1.97)b |
| >5 | 4.58 (3.07–6.84)b |
| Fibroids | |
| Yes | 0.87(0.71–1.07) |
| No | Reference |
| Endometriosis | |
| Yes | 1.48 (1.19–1.83)b |
| No | Reference |
| Abnormal menstruation bleeding | |
| Yes | 0.80 (0.68–0.95)b |
| No | Reference |
| Benign neoplasm or cyst | |
| Yes | 1.07 (0.88–1.30) |
| No | Reference |
| Pelvic organ prolapse | |
| Yes | 0.66 (0.44–0.99) |
| No | Reference |
CI, confidence interval; MIS, minimally invasive surgery.
Multivariable regression adjusted for operative technique (MIS vs open), surgeon hysterectomy volume, hospital hysterectomy volume, hospital bed size, surgeon practicing year, patient age, patient race, payer, Elixhauser score, and all 5 selected benign indications;
Odds ratios and 95% confidence intervals represent significant values.
Mehta et al. Disparities associated with benign hysterectomy approach and complications. Am J Obstet Gynecol 2017.
FIGURE 3. Proportion of physicians performing only open hysterectomies by surgeon volume group.
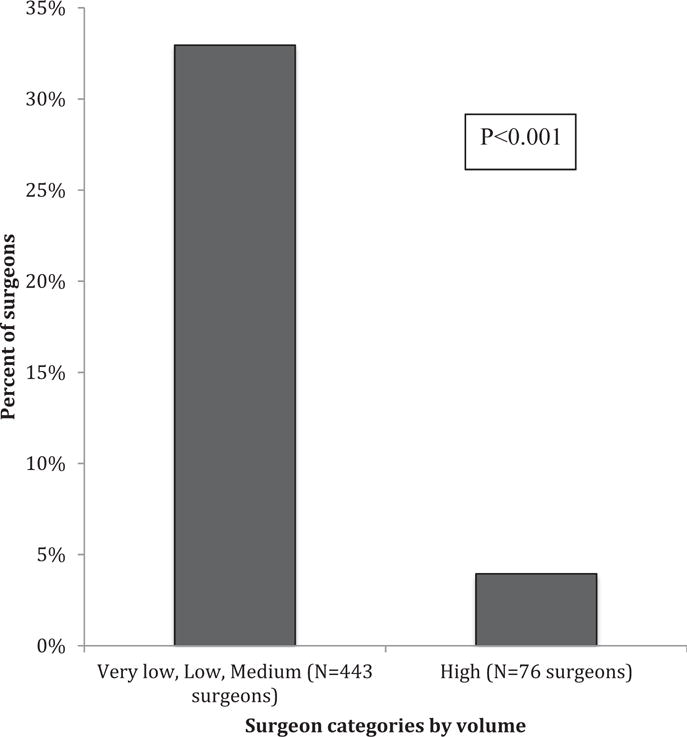
Mehta et al. Disparities associated with benign hysterectomy approach and complications. Am J Obstet Gynecol 2017.
Comment
Variation in MIS is suggested to represent one of the greatest disparities in medicine.20 Our study utilizing a state-specific, all-payer data set demonstrates several concerning practices of hysterectomy surgery for benign disease. Surgeon preference to perform minimally invasive hysterectomy compared with open abdominal hysterectomy surgery remains highly variable, even after adjusting for differences in patient diagnosis and comorbidity profile. Despite ample level-I evidence supporting the superiority of minimally invasive hysterectomy for the treatment of many benign gynecologic conditions,10 the 2012 through 2014 rate of open abdominal hysterectomy in the state of Maryland remain alarmingly high (61.5%). Several factors were associated with open hysterectomy including advancing patient age, nonwhite race, small and medium hospital, and medium- compared with higher-volume surgeons. Although all hysterectomies cannot be performed via minimally invasive approaches, and the database used does not include data on uterine size and other factors that may impact surgical approach, previous studies demonstrate that the majority of hysterectomies can be completed vaginally or laparoscopically when performed by experienced, high-volume surgeons.2,14 Despite this, the vast majority (68.2%) of the 618 Maryland physicians performing hysterectomies in the current study completed <10 hysterectomies during the 2-year study period, and their patients developed more than double the number of perioperative complications when compared to those operated on by higher-volume surgeons. These data add to the growing body of evidence that suggests that a centralized model (when feasible) with higher-volume surgeons performing hysterectomy surgery and/or assisting lower-volume surgeons, may be associated with improved outcomes and lower complication rates.
In several surgical disciplines, the relationship between surgical volume and outcome is well recognized. A US study of 470,000 Medicare patients undergoing either cardiovascular procedures or cancer resections found that the operative mortality rate was strongly and inversely related to surgeon volume for each procedure.21 In gynecology, a 2016 review of surgeon volumes and outcomes for benign hysterectomy concluded that morbidity was higher for low-volume surgeons and that high-volume surgeons were more efficient.22 Additionally, a recent systematic review of 14 peer-reviewed international studies of gynecology, gynecologic oncology, and urogynecologic patients undergoing hysterectomy was undertaken to determine the impact of surgeon volume on patient outcomes and found that the cohort of low-volume surgeons (≤12 hysterectomies) had an increased rate of total, intraoperative, and postoperative complications. Despite these compelling data in support of high-volume surgeon practice models, our study shows that as recently as 2014, more than two thirds of all hysterectomies in the state of Maryland were performed by low-volume surgeons.
Recently, shifts have occurred in nationwide surgical referral patterns based on public reporting of outcomes and quality initiatives. Efforts have focused on concentrating specialized procedures and/or high-risk patients to high-volume facilities and centers of excellence to reduce the risk of perioperative adverse events.3 To our knowledge, this more centralized care model has not been considered for hysterectomy surgery, although our study and others suggest that this merits attention. Our findings provide rationale for utilizing hysterectomy surgeon volume as a quality indicator. If higher-volume surgeons operated on all of the patients in the current study, a substantial proportion of perioperative complications and their associated health care costs may have been averted. It is important to note, however, that volume is not the sole surrogate for patient safety and quality, and quality improvement measures should also incorporate a surgeon’s cumulative experience, which our database did not capture. Furthermore, the number of cases that define a “high-volume” hysterectomy surgeon is unclear and requires further study.
The importance of surgeon volume compared with hospital volume in measuring surgical patient outcomes has been oft debated. Databases such as the Maryland HSCRC that offer insight into both surgeon and hospital-based outcomes allow for a potentially more nuanced assessment of a clinical problem. While surgeon and hospital volume may appear to be collinear variables, that is not always the case, and there is value to studying both variables, when available. For instance, although most physicians in our study were very low– or low-volume surgeons, almost half practiced at high hysterectomy–volume hospitals. It is possible that low-volume surgeons who practice at high-volume centers may have more opportunity to consult with their higher-volume counterparts in the surgical care of their patients, although we do not have the means within our state registry data to examine this question. However, several studies imply that low-volume surgeons who operate at higher-volume centers may have better outcomes than low-volume surgeons operating at low-volume hospitals, known as a “field effect” phenomenon.22 Conversely, studies also demonstrated that high-volume surgeons who operate at lower-volume hospitals may produce excellent outcomes.23,24 Most national and statewide surgical registries do not include surgeon-specific data, and our study is unique in providing outcomes based on both surgeon and hospital volume. From an epidemiologic perspective, reducing hysterectomy-based preventable harm in the United States may depend upon optimizing not only where a patient is operated on but by whom. Potential solutions to address inequalities in receipt of MIS performed by low-volume physicians include: (1) increasing locoregional and national minimally invasive training opportunities for OB/GYN residents, fellows, and practicing gynecologists; (2) consideration of more referrals to high-volume, experienced hysterectomy surgeons with lower complication rates; and (3) consolidation of surgical care within OB/GYN physician group practices and academic departments, with fewer physicians performing hysterectomies and/or more experienced surgeons on hand to assist lower-volume surgeons with their surgical cases. The latter option may be the most realistic, near-term solution to make an immediate impact on patient outcomes after major gynecologic surgery.
Several patient factors were also associated with surgical outcome in our analysis, including race and socioeconomic status. Black patient race (compared to white), Hispanic ethnicity, and payer status (those with no insurance, Medicaid or Medicare) were independently associated with perioperative complications after hysterectomy, while age, black or other patient race, and receiving care at a small hospital were associated with underutilization of MIS hysterectomy. Our findings suggest a racial and socioeconomic disparity in receiving quality gynecologic surgical care that is also observed in other studies.15,25 The cause of racial and ethnic differences with regards to hysterectomy approach and operative outcomes is not fully understood. Acomplex set of genetic, physiologic, socioeconomic, and cultural factors likely contribute to racial and ethnic disparities, as has been described in other fields of medicine.26 For instance, it is well understood that black women are 3 times more likely to develop uterine leiomyomas requiring surgery than white women,26 and this factor may have contributed to the greater rates of open hysterectomy surgery observed in black women in our study. Moreover, differences in patient preferences by race and ethnicity may also influence treatment decisions resulting in differential rates of surgery. Nevertheless, more black women and uninsured women experienced perioperative complications in the current study that may be related to receiving care at smaller hospitals with lower-volume surgeons. In an epidemiologic study of 2000 women undergoing hysterectomy surgery in California, Michigan, and Georgia in 1991, Medicaid-covered women (40%) and black women (68%) were more likely to experience a postoperative complication compared with privately insured women and white women, respectively.27 Both subsets of women in this study were also more likely to undergo the open, abdominal hysterectomy than white or privately insured women. The direct causes for the increased complications rates observed in these cohorts were unclear, but were possibly related to surgical approach, to a delay in access of care prior to the hospitalizations, and to patient-related comorbidities. It is disturbing that >2 decades later, similar trends in surgical outcome are observed in our study based on race and socioeconomic status. Prospective trials focused on receipt of appropriate hysterectomy care and patient- and hospital-specific factors that impact this care are needed so that these disparities may be addressed.
Despite the inclusion of a large patient cohort, we recognize some important limitations. As with all claims data sets, there may be missing variables within the medical records. Classification of hysterectomy is based only on ICD-9-CM coding and, therefore, we cannot exclude the possibility that the type of procedure performed was miscoded in a small number of women (although this misclassification would have minimal effect given the large patient sample in our study). Likewise, our study lacks data on clinical characteristics likely to influence the route of surgery, including prior surgical procedures, pathology, uncoded patient health factors (ie, body mass index), and other uterine factors, such as uterine size. In addition, we could not apply necessary weights to approximate nationwide utilization of MIS for hysterectomies and average perioperative complications by surgeon volume. However, our statewide data are congruent with national surgical trends.14 Finally, the majority of national surgical databases only provide inpatient hospital data focused on patient- and hospital-specific factors, and omits surgeon-specific data. The strength of examining a statewide registry such as the Maryland HSCRC is the inclusion of all hysterectomy procedures performed during a predetermined period (including same-day, outpatient, and inpatient procedures) and the inclusion of surgeon-specific variables that may more directly inform opportunities for quality improvement.
Our data demonstrate substantial inequalities in gynecologic surgical care according to patient, surgeon, and hospital characteristics, and identifies an important opportunity for quality improvement, with the potential to reduce preventable harm and associated health care costs. Efforts to incentivize referrals to higher-volume hysterectomy surgeons and to increase the number of minimally invasive hysterectomy procedures should be a priority within the gynecologic surgical community, both in Maryland and nationwide. Additionally, improving education and access to quality gynecologic surgical care for all women, irrespective of age, comorbidities, race, or payer status, is paramount. Both utilization of MIS and surgeon hysterectomy volume may serve as metrics for quality improvement initiatives and cost-saving measures. A study is planned at our institution to further study these potential quality measures.
Footnotes
The authors report no conflict of interest.
References
- 1.Dessources K, Hou JY, Tergas AI, et al. Factors associated with 30-day hospital readmission after hysterectomy. Obstet Gynecol. 2015;125:461–70. doi: 10.1097/AOG.0000000000000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen SL, Vitonis AF, Einarsson JI. Updated hysterectomy surveillance and factors associated with minimally invasive hysterectomy. JSLS. 2014;18(3) doi: 10.4293/JSLS.2014.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122:233–41. doi: 10.1097/AOG.0b013e318299a6cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–9. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Cui J, Jia L, Hong S, Kong B, Li D. Comparison of laparoscopy and laparotomy for endometrial cancer. Int J Gynaecol Obstet. 2012;116:185–91. doi: 10.1016/j.ijgo.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Kiran RP, El-Gazzaz GH, Vogel JD, Remzi FH. Laparoscopic approach significantly reduces surgical site infections after colorectal surgery: data from national surgical quality improvement program. J Am Coll Surg. 2010;211:232–8. doi: 10.1016/j.jamcollsurg.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Palomba S, Falbo A, Mocciaro R, Russo T, Zullo F. Laparoscopic treatment for endometrial cancer: a meta-analysis of randomized controlled trials (RCTs) Gynecol Oncol. 2009;112:415–21. doi: 10.1016/j.ygyno.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Sauerland S, Jaschinski T, Neugebauer EA. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2010;(10):Cd001546. doi: 10.1002/14651858.CD001546.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Galaal K, Bryant A, Fisher AD, Al-Khaduri M, Kew F, Lopes AD. Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database Syst Rev. 2012;9:Cd006655. doi: 10.1002/14651858.CD006655.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynecological disease. Cochrane Database Syst Rev. 2009;(3):Cd003677. doi: 10.1002/14651858.CD003677.pub4. [DOI] [PubMed] [Google Scholar]
- 11.Aimaq R, Akopian G, Kaufman HS. Surgical site infection rates in laparoscopic versus open colorectal surgery. Am Surg. 2011;77:1290–4. doi: 10.1177/000313481107701003. [DOI] [PubMed] [Google Scholar]
- 12.Scalici J, Laughlin BB, Finan MA, Wang B, Rocconi RP. The trend towards minimally invasive surgery (MIS) for endometrial cancer: an ACS-NSQIP evaluation of surgical outcomes. Gynecol Oncol. 2015;136:512–5. doi: 10.1016/j.ygyno.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Wright JD, Neugut AI, Wilde ET, Buono DL, Tsai WY, Hershman DL. Use and benefits of laparoscopic hysterectomy for stage I endometrial cancer among Medicare beneficiaries. J Oncol Pract. 2012;8:e89–99. doi: 10.1200/JOP.2011.000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aarts JW, Nieboer TE, Johnson N, et al. Surgical approach to hysterectomy for benign gynecological disease. Cochrane Database Syst Rev. 2015;8:Cd003677. doi: 10.1002/14651858.CD003677.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fader AN, Weise RM, Sinno AK, et al. Utilization of minimally invasive surgery in endometrial cancer care: a quality and cost disparity. Obstet Gynecol. 2016;127:91–100. doi: 10.1097/AOG.0000000000001180. [DOI] [PubMed] [Google Scholar]
- 16.Agency for Healthcare Research and Quality. Patient safety indicators overview. Available at: http://www.qualityindicators.ahrq.gov/Modules/psi_resources.aspx. Accessed Jan. 10, 2016.
- 17.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Rhee C, Huang SS, Berrios-Torres SI, et al. Surgical site infection surveillance following ambulatory surgery. Infect Control Hosp Epidemiol. 2015;36:225–8. doi: 10.1017/ice.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.UCLA Institute for Digital Research and Education. Stata data analysis examples– negative binomial regression. Available at: http://www.ats.ucla.edu/stat/stata/dae/nbreg.htm. Accessed Aug. 10, 2016.
- 20.Cooper MA, Hutfless S, Segev DL, Ibrahim A, Lyu H, Makary MA. Hospital level under-utilization of minimally invasive surgery in the United States: retrospective review. BMJ. 2014;349:g4198. doi: 10.1136/bmj.g4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–27. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 22.Wood TW, Ross SB, Bowman TA, et al. High-volume hospitals with high-volume and low-volume surgeons: is there a “field effect” for pancreaticoduodenectomy? Am Surg. 2016;82:407–11. [PubMed] [Google Scholar]
- 23.Toomey PG, Teta AF, Patel KD, Ross SB, Rosemurgy AS. High-volume surgeons vs high-volume hospitals: are best outcomes more due to who or where? Am J Surg. 2016;211:59–63. doi: 10.1016/j.amjsurg.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Doll KM, Milad MP, Gossett DR. Surgeon volume and outcomes in benign hysterectomy. J Minim Invasive Gynecol. 2013;20:554–61. doi: 10.1016/j.jmig.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Hakim RB, Benedict MB, Merrick NJ. Quality of care for women undergoing a hysterectomy: effects of insurance and race/ethnicity. Am J Public Health. 2004;94:1399–405. doi: 10.2105/ajph.94.8.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahdi H, Lockhart D, Moslemi-Kebria M, Rose PG. Racial disparity in the 30-day morbidity and mortality after surgery for endometrial cancer. Gynecol Oncol. 2014;134:510–5. doi: 10.1016/j.ygyno.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Jacoby VL, Fujimoto VY, Giudice LC, Kuppermann M, Washington AE. Racial and ethnic disparities in benign gynecologic conditions and associated surgeries. Am J Obstet Gynecol. 2010;202:514–21. doi: 10.1016/j.ajog.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]


