Figure 1.
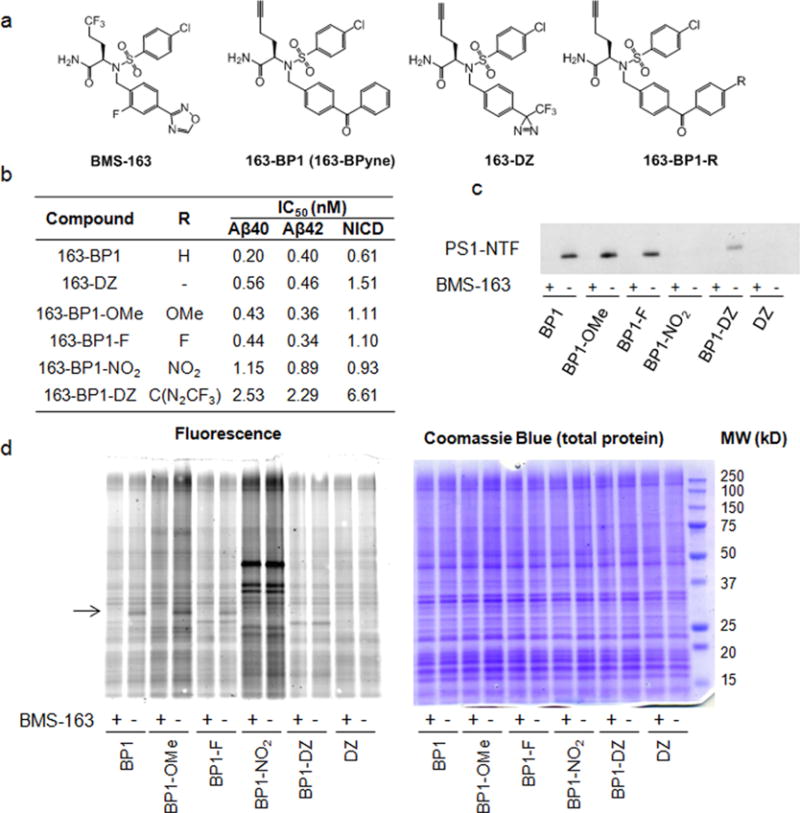
Comparison of photoreactive groups and para substituted benzophenones for labeling PS1–NTF. (a) Chemical structures of probes. (b) IC50 values of these probes against γ-secretase for Aβ40, Aβ42, and NICD1 production. (c) Comparison of PS1–NTF labeling efficiency. Probes (20 nM) were incubated with HeLa membranes with and without 2 μM competitor BMS-163, UV-irradiated to cross-link, and tagged with biotin azide via click chemistry, followed by pull-down with streptavidin and Western blot for PS1–NTF. (d) Probes (20 nM) were incubated with HeLa membranes with and without 2 μM competitor BMS-163, UV-irradiated to cross-link, and tagged with TAMRAZ–azide (see Supplementary Figure 2 for the structure of TAMRA–azide) via click chemistry, followed by in-gel fluorescence (left). Coomassie blue staining (right) shows equal amounts of total protein loaded on the gel. The arrow points to PS1–NTF at approximately 30 kDa.
