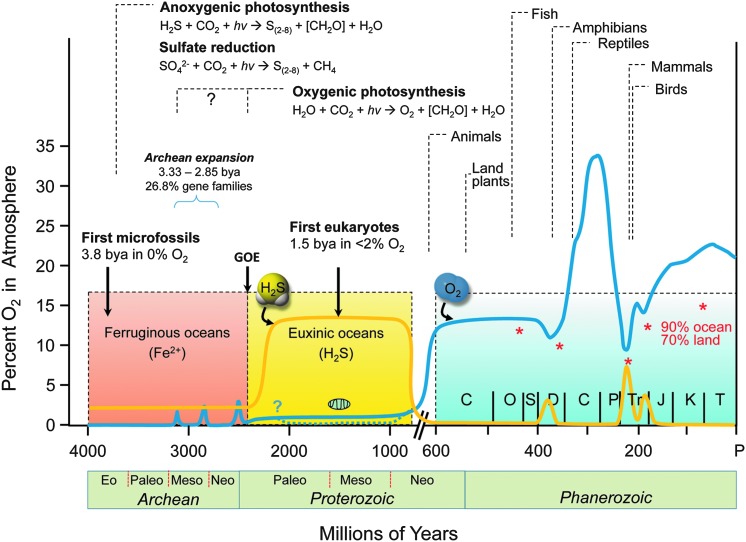
FIG. 2.
Evolution of sulfur and oxygen metabolism. The lines indicate fluctuations in concentration of atmospheric oxygen (blue) and oceanic sulfide (orange) over evolutionary times. Atmospheric O2 was essentially absent from the environment at the onset of life ∼3.8 bya. After the great oxidation event (GOE), the concentration of O2 in the atmosphere increased, which was accompanied by a substantial increase in H2S. The first eukaryotes appeared in oceans and developed in anoxic and sulfidic (euxinic) conditions for hundreds of millions of years using sulfur as their energy source, producing RSS. During this time, defense mechanisms against RSS evolved, improving cell survival and minimizing the need for repair of damaged cell constituents. Appearance of oxygenic cyanobacteria and plants led to increases in O2 levels and oxidation of H2S and Fe2+ ∼0.6 bya. Those changes were accompanied by a significant decrease in dissolved H2S and a repurposing of enzymatic systems that originally evolved to protect organisms against RSS to serve additional antioxidative protective functions. Mass extinctions (*, percentage of marine and land life) were often associated with a fall in ambient O2 and increases in H2S, perhaps providing a biological filter for descendants that retained some degree of tolerance to hypoxia and sulfide. Modified with permission from Olson and Straub (139). bya, billion years ago; H2S, hydrogen sulfide.