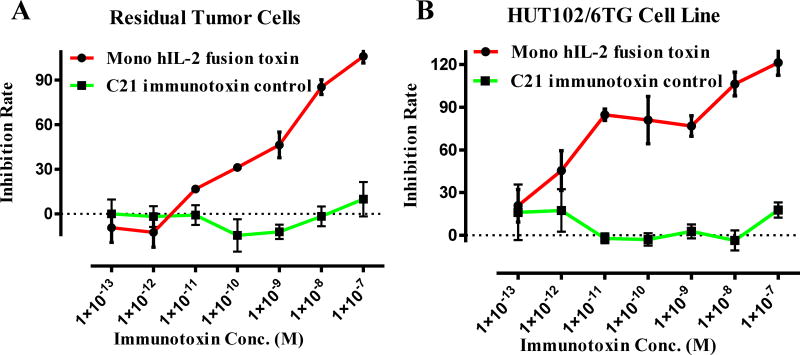
Fig. 6.
In vitro efficacy analysis of the monovalent human IL-2 fusion toxin to the residual tumor cells using CellTiter-Glo® Luminescent Cell Viability Assay (Promega, cat# G7571). A) To the residual tumor cells: 1) monovalent human IL-2 fusion toxin (red line); 2) C21 immunotoxin as negative control (black line). B) To the HUT102/6TG cell line: 1) monovalent human IL-2 fusion toxin (red line); 2) C21 immunotoxin as negative control (black line). Y-axis: inhibition rate of the cell viability by determining the number of viable cells based on the quantification of the ATP present. X-axis: plated human IL-2 fusion toxin concentration. Cycloheximide (1.25 mg/mL) was used as a positive control. The negative control contained cells without immunotoxin. P < 0.0001 by Log-rank (Mantel-Cox) Test of Prism (n=3). Error bars indicate ±SD. Data are representative of three assays.