Figure 4.
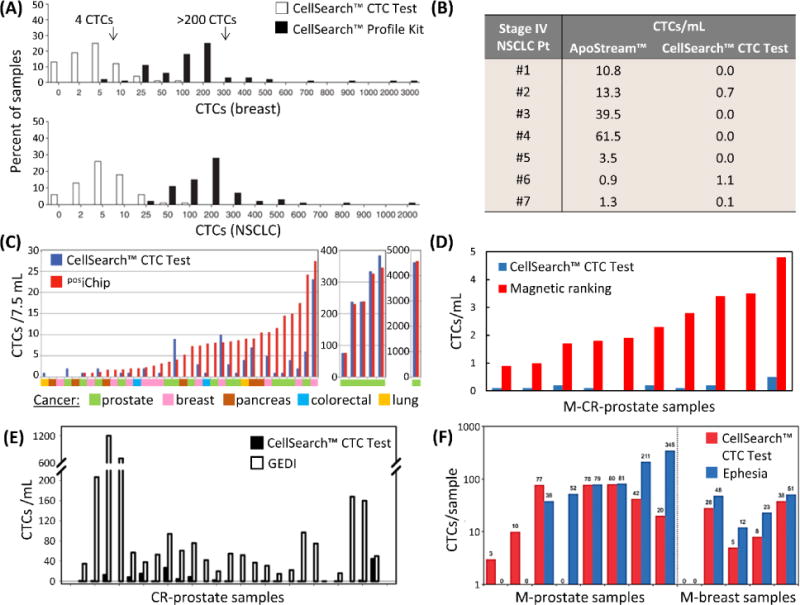
Direct comparisons to the CellSearch™ CTC Test by (A) the CellSearch™ Profile Kit,78 (B) Apostream™,214 (C) the posiChip,107 (D) the magnetic ranking microfluidic device,118 (E) the GEDI micropillar device,73 and (F) the Ephesia microfluidic device.60 Note that magnetic ranking and Ephesia technologies collected blood in CellSave™ tubes in comparisons,60,118 and the GEDI device selected PSMA(+) CTCs, whereas the CTC Test targeted EpCAM(+) CTCs. In this study, Kirby et al. noted that 60% (median) of CTCs were PSMA(+)/EpCAM(+), indicating the GEDI yields were roughly 10-fold greater than by the CellSearch™ CTC Test.73 Figure panels reproduced from reference78 with permission from Nature Publishing Group, copyright 2010; reference107 with permission from The American Association for the Advancement of Science, copyright 2013; and reference118 with permission from Nature Publishing Group, copyright 2017.
