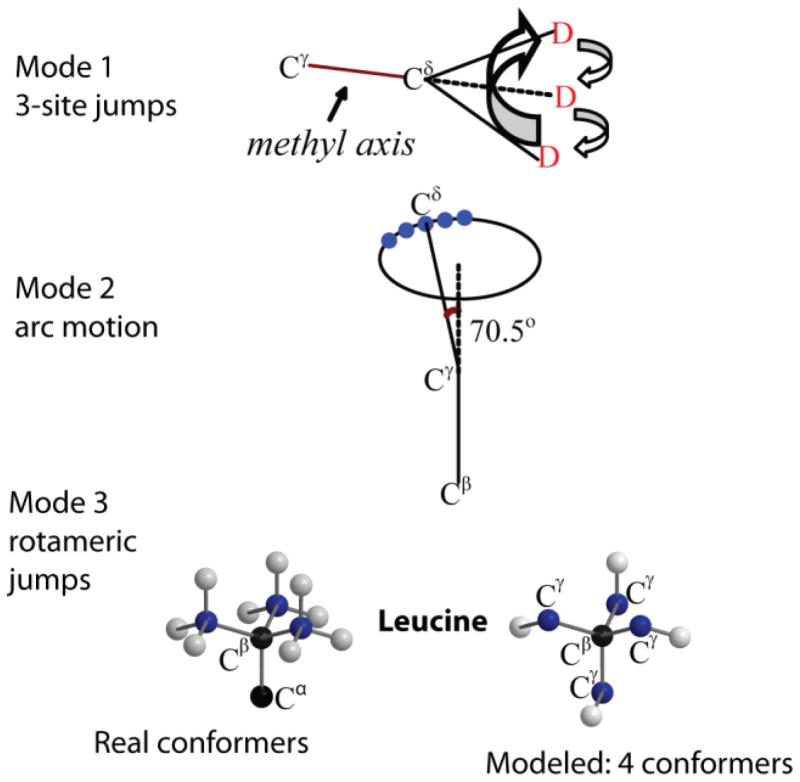
Figure 24.
Schematic representation of the motional model used to fit the line shapes of the hydrophobic core leucine side-chains in the villin headpiece subdomain protein.(29, 40) The first mode corresponds to the fast 3-site jumps of the methyl group; the second mode corresponds to a restricted diffusion on an arc approximated by small nearest neighbor jumps of the Cγ–Cδ axis; the third mode represents the mode of the rotameric jumps corresponding to hops between four non-equivalent positions of the leucine side-chain out of the nine possible configurations. Adapted with permission from (29). Copyright (2011) American Chemical Society.